Concept about Matter – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Concept about Matter
In the classical physics and in the chemistry of things of everyday life, matter is any substance that has mass and takes up space by having volume. All everyday objects that we can touch are ultimately composed of atoms, which are made up of interacting subatomic particles, and in everyday as well as scientific usage, “matter” generally includes atoms and anything made up of them, and any particles (or combination of particles) that act as if they have both rest mass and volume.
However it does not include massless particles such as photons, or other energy phenomena or waves such as light or sound. Matter exists in various states (also known as phases). These include classical everyday phases such as solid, liquid, and gas for example water exists as ice, liquid water, and gaseous steam but other states are possible, including plasma, Bose-Einstein condensates, fermionic condensates, and quark-gluon plasma.
Molecules are composed of atoms. Molecules vary in size from those composed of two atoms such as molecules of O2 (oxygen) or H2 (hydrogen), to those composed of thousands of atoms, such as organic molecules of proteins.
Human life is dependent not only upon the small molecules of O2 and H2O (water) but also upon the large molecules of proteins, which are necessary for the processes of metabolism, assimilation, and propagation of the human race. A definite piece of matter is called ‘Body’ e.g., minute bodies like grains of sand whereas large bodies like the Sun, or Earth.
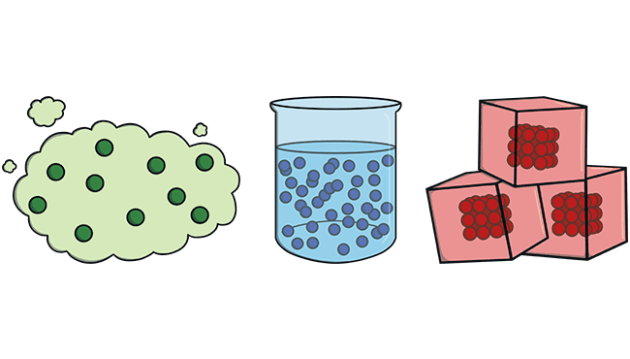
Examples of Matter
Matter can include any of the following (and more, of course):
- Proton
- Atoms (e.g., a helium atom)
- Molecules (e.g., water, sugar)
- Compounds (e.g., table salt, silicon dioxide)
- Cat
- Tree
- House
- Computer
Examples that are not matter
Not everything we can perceive consists of matter. Examples of things that aren’t matter include:
- Photons (light)
- Heat
- Thoughts
- Microwaves (the radiation, not the appliance)

Definition of Matter:
Matter is defined as anything that occupies space and has mass. It is made up of molecules in constant erratic motion.
or
Matter is anything that takes up space and can be weighed. In other words, matter has volume and mass.
or
In the classical physics and in the chemistry of things of everyday life, matter is any substance that has mass and takes up space by having volume.

Read More….