Diffusion and Osmosis – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Diffusion and Osmosis
Diffusion:
The continuous movement of molecules in a solution from a higher concentration to a lower concentration (i.e along the conc. Gradient) is called Diffusion. The energy that causes diffusion is the energy of the normal kinetic motion of the solute molecules.
or
Diffusion may be regarded as the movement of solute molecules by the normal kinetic motion from a higher concentration to a lower concentration.
Types of Diffusion with example:
- Simple diffusion: Here the movement of molecules occurs without the help of carrier protein. e.g- O2, CO2 transport etc
- Facilitated diffusion: Here the movement of molecules occur with the help of carrier protein. e.g- Glucose transport.
Ref- Guyton & Hall/13th/48)
Factors affecting the rate of diffusion:
- Thickness of the cell membrane:↑ thickness rate of diffusion.
- Lipid solubility: ↑Lipid solubility ↑rate of diffusion.
- Number of protein channel: ↑number Trate of diffusion.
- Concentration gradient.
- Temperature: ↑Temperature rate of diffusion.
- Electrical potential.
- Molecular weight: ↑Molecular weight→↑ rate of diffusion
In an another way
The factors that affect rate of diffusion are as follows-
➤ Concentration difference: Rate of diffusion x Concentration difference (AC).
➤ Cross sectional area (A): Rate of diffusion Cross sectional area (A),
➤ Distance between two regions (AX):
- Rate of diffusion x = 1/ Distance between two regions
➤ Temperature: ↑ Temperature →↑ Kinetic energy→↑ Rate of diffusion.
➤ Size of particles: ↑ Size Rate of diffusion.
➤ Molecular weight of the particles: ↑ MW→↑ rate of diffusion.
➤ Thickness of the cell membrane: ↑ thickness rate of diffusion
➤ Lipid solubility: ↑ lipid solubility→↑rate of diffusion.
➤ Number of protein channels in the cell membrane: ↑ number →↑ rate of diffusion.
Importance of Diffusion:
- Admixture of foodstuff with digestive juices.
- Absorption of various substances from the intestine.
- Exchange between plasma & red cells.
- Exchange in the capillary beds.
- Exchange of gases in the lungs.
- Excretion of waste products from the kidney tubules.
In an another way
Physiological importance of Diffusion:
- Exchange of O₂ & CO₂ in lungs and tissues occurs by diffusion.
- Absorption of water soluble vitamins, minerals etc from GIT occurs by diffusion.
- Exchange in the capillary occurs by diffusion.
- Secretion of waste product (e.g. ammonia) into renal tubules occurs by diffusion.
- Diffusion of Na & K’ through Na – K’ leak channel creates resting membrane potential.
- Pneumonia → ↑thickness of alveolar membrane diffusion of O2 → hypoxia.
Fick’s Law of Diffusion:
The magnitude of diffusing tendency from one region to another is proportionate to the difference in the concentration of the substances in the two regions (conc. Gradient), the cross sectional area of the boundary across which diffusion is taking place and the thickness of the boundary.
Thus,
Rate of diffusion (J) = -DA (AC/Ax)
Here, D = diffusion coefficient.
A = cross sectional area.
AC/Ax = concentration gradient.
Another Answer:
According to the Fick’s law of
diffusion-
Rate of diffusion (J),
➤ J=-DA AC/AX
Where,
- J – is the net rate of diffusion,
- D – is the diffusion coefficient,
- A – is the area,
- Ac – is the concentration difference,
- Ax – is the distance between two regions, and
- AC/Ax – is the concentration gradient. The minus sign indicates the direction of diffusion.
When considering movement of molecules from a higher to a lower concentration, Ac/Ax is negative, so multiplying by -DA gives a positive value.
- Thickness of the membrane: inversely proportional to diffusion.
- Surface area of the membrane: directly proportional to diffusion.
- Pressure difference: directly proportional to diffusion.
- Diffusion coefficient (D): directly proportional to diffusion.
(Ref-Ganong/25th/6+ Guyton & Hall /12th/492)
Are various processes that produce movement of substances across the cell membrane with an example of each
- Simple diffusion: Oxygen transport.
- Facilitated diffusion: Glucose transport
- Primary active transport: Calcium ion transport.
- Secondary active transport:
✓ Co-transport: Na-glucose co transport
✓ Counter transport: Na-hydrogen counter transport
Diffusion through cell membrane:
Diffusion through the cell membrane is divided into two subtypes –
➤ Simple diffusion
➤ Facilitated diffusion.
Simple diffusion:
➤ Simple diffusion means that kinetic movement of molecules or ions occurs through a membrane opening or through intermolecular spaces without any interaction with carrier proteins in the membrane.
➤ The rate of diffusion is determined by the amount of substance available, the velocity of kinetic motion, and the number and sizes of openings in the membrane through which the molecules or ions can move.
➤ Simple diffusion can occur through the cell membrane by two pathways:
- Through the interstices of the lipid bilayer if the diffusing substance is lipid soluble, and
- Through watery channels that penetrate all the way through some of the large transport proteins
Facilitated diffusion:
➤ Facilitated diffusion requires interaction of a carrier protein. The carrier protein aids passage of the molecules or ions through the membrane by binding chemically with them and shuttling them through the membrane in this form.
(Ref- Guyton & Hall/13th/48)
Transport of Glucose:
Transport of Glucose into the cell occurs by the process of-
- Active transport.
- Facilitated diffusion.
- There are two types of transport:
➤ Insulin independent: It occurs in
- Liver.
- Brain.
- Spleen.
- RBC & WBC
- Cornea.
- Lens of eye
➤ Insulin dependent: It occurs in –
- Adipose tissues.
- Skeletal muscles etc

Differentiate between diffusion & osmosis
Difference between diffusion & osmosis:
| Traits | Diffusion | Osmosis |
| Types of movement | Movement of the solute molecules occur | Movement of solute molecules occur |
| Direction of movement | From higher to lower concentration | From lower to higher concentration |
| Semipermeable membrane | May or may not be present | Must be present |
| Permeability of the membrane | Permeable to solute & solvent | Permeable to solvent only |
| Equality of hydrostatic pressure | It must be equal on two sides | Not necessary |
| Duration | It occurs at every moment | It continues as long as osmotic pressure exists |
| Depends on | Concentration gradient, molecular wt. of the solute particles, cross sectional area and temperature | Number of solute particles per unit volume and temperature |
Differentiate between simple & facilitated diffusion.
Differences between simple & facilitated diffusion:
| Traits | Simple diffusion | Facilitated diffusion |
| Definition | Movements of the molecules from higher to lower concentration or id towards the electrical or chemical gradient without carrier & energy is called simple diffusion. | Movements of the molecules from higher to lower concentration or towards the electrical or chemical gradient with the help of carrier protein is called facilitated diffusion |
| Carrier | Not needed | Needed |
| Saturation of carrier | Not occur | Saturation of carrier protein occur |
| Diffusion capacity | Unlimited | Limited (due to saturation of carrier) |
| Substances that are transported | Lipid soluble | Lipid insoluble |
| Example | O2, N2, CO2, Alcohol, H₂O etc | Glucose, Amainoacid, Galactose etc |
Differences between active transport and passive transport/diffusion:
| Traits | Active transport | Passive transport |
| 1. Movement occurs | Against concentration or electrical gradient. i.e- uphill movement. | Towards the concentration or electrical gradient. i.e- downhill movement. |
| 2. Energy | Required | Not required. |
| 3. Carrier protein | Required | Required for facilitated diffusion but not for simple diffusion and osmosis |
| 4. Membrane | Must be present | May or may not be present. |
| 5. Saturation of carrier | Occurs | Only in facilitated diffusion, saturation of carrier occurs. |
| 6. Direction of movement | Usually unidirectional | Can operate bi-directional. |
| 7. Role of electrochemical gradient | Does not depend on them | Depends on them. |
| 8. Dependability | Depends upon the availability of enzyme & carrier | Depends upon conc. gradient, hydrostatic pressure & osmotic pressure. |
Differences between active transport & facilitated diffusion:
| Traits | Active transport | Facilitated diffusion |
| 1. Movement occurs | Against the concentration & electrochemical gradient. | Towards the concentration & electrochemical gradient |
| 2. Energy | Required | Not required. |
| 3. Presence of membrane | Must be present. | May or may not be present. |
| 4. Role of electrochemical gradient | Does not depend on them | Depends on them. |
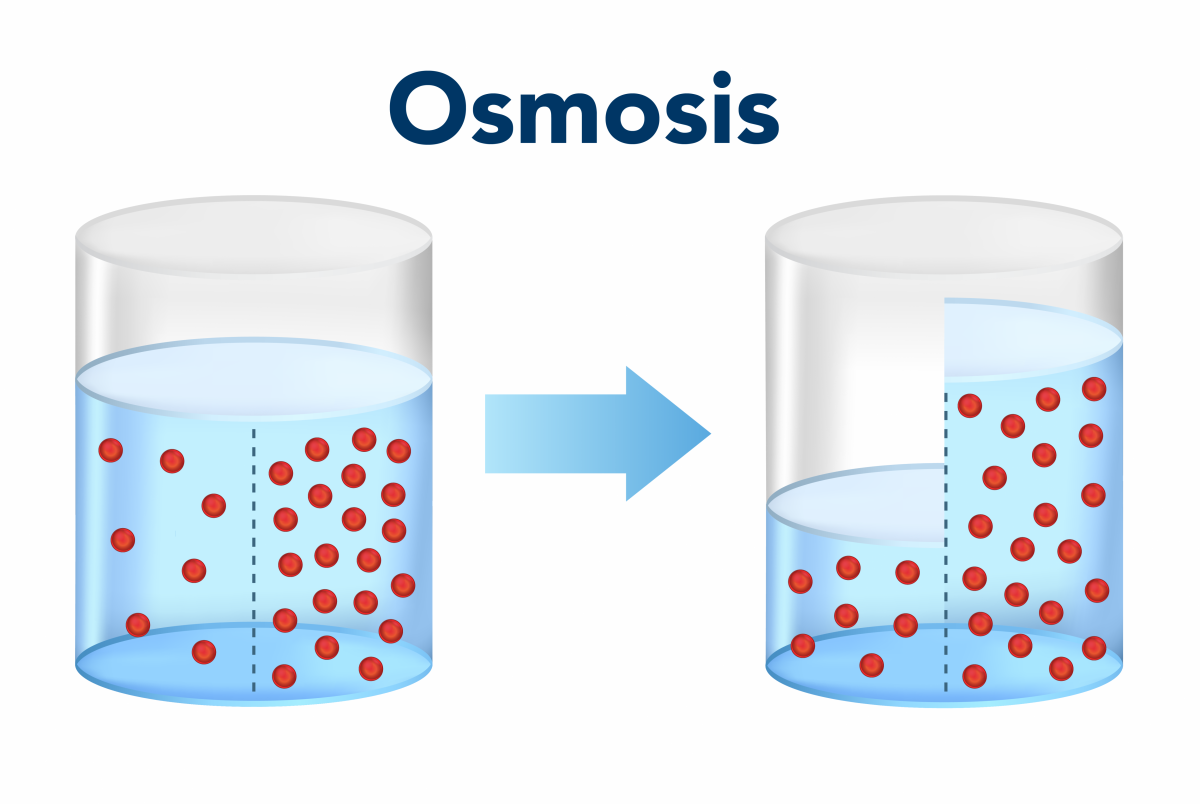
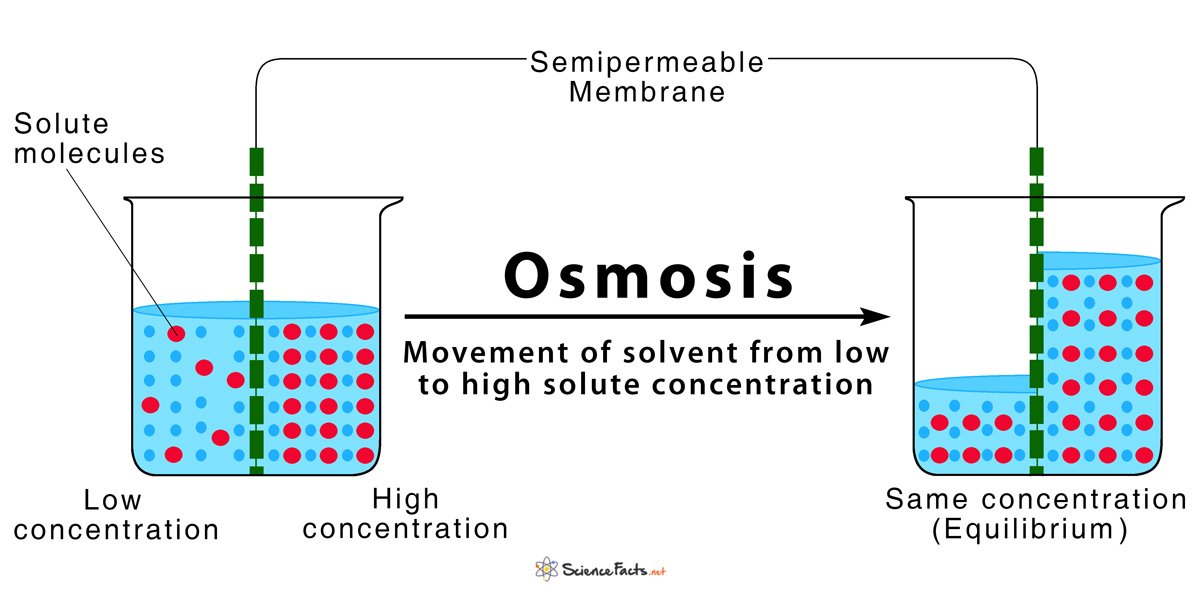
Osmosis
The migration of solvent molecules from the region of lower solute concentration to the region of higher solute concentration across a semipermeable membrane is called osmosis.
Condition of osmosis:
- Two solution of different osmotic pressure.
- Presence of a semipermeable membrane.
Importance of osmosis:
A. Physiological:
- Absorption from GIT
- Fluid exchange in the capillary bed.
- Regulation of urine formation.
- Reabsorption of CSF
- Maintenance of Blood pressure.
B. Clinical:
- Infusion: Isotonic solutions of NaCI (0.9%) or glucose (5%) are commonly used as infusion in hospitals for the treatment of dehydration, burn etc.
- Action of Purgatives: Purgatives (e.g. MgSO4) prevent water absorption by osmosis from GIT. Thus they cause dilution of intestinal content and helps in defecation.
- Osmotic diuresis: The high blood glucose concentration (e.g. in Diabetes mellitus) causes osmotic diuresis, resulting in the loss of water, electrolytes and glucose in the urine.
- Edema due to hypoalbuminaemia: When plasma protein is decreased, water leaves blood vessels and enter into the interstitial space by osmosis, thus edema develops.
- Cerebral edema: In cerebral edema, hypertonic solution is used that causes osmosis of water from brain and thus prevents edema.
- Irrigation of wounds: Isotonic solutions are used for washing wounds. The pain experienced by the direct addition of salt or sugar to the wounds is due to osmotic removal of water
(Ref: Satyanarayana/3rd/713-14p)
Osmotic pressure:
The amount of pressure required to prevent osmosis completely is called osmotic pressure. Osmotic pressure causes fluid movement by osmosis from the interstitial space into the blood. It is measured as Osmole/L or milliosmole/L. The plasma colloidal osmotic pressure is 280-300 mOsm/L.
N.B: The opposite of osmotic pressure is ‘hydrostatic pressure’. Hydrostatic pressure causes movement of solute & fluid from the capillary blood to the interstitial space.]
Colloidal osmotic pressure (COM):
The osmotic pressure that is exerted by the colloids is called colloidal osmotic pressure. Plasma colloidal osmotic pressure is the osmotic pressure exerted by the plasma proteins. COM causes fluid movement from the interstitial space to the blood by osmosis.
Importance:
- COM prevents loss of fluid from the blood into the interstitial space.
- COM causes osmosis of fluid inward through the capillary membrane. Thus it prevents formation of edema.
- It controls the intracellular fluid volume as well as interstitial fluid volume.
- It plays an important role in the regulation of the glomerular filtration.
- ↓COM results in the development of edema.
(Ref: Guyton and Hall/ 12th 181)

Read More….