Trace Elements – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Trace Elements
These are elements required by the body in quantities of less than a few milligrams per day” e.g., iron, iodine, fluorine, zinc, copper, cobalt, chromium, manganese, molybdenum, selenium, nickel, tin, silicon and vanadium.
or
It is metals and other elements that are regularly present in very small amount in the tissue and to be essential for normal metabolism. E.g., copper, cobalt, fluorine, iron, iodine etc.
Importance of Trace Element for Human Health:
➤ Calcium: It intervenes in the nervous system. Additionally, it intervenes with bones, teeth, and blood clotting.
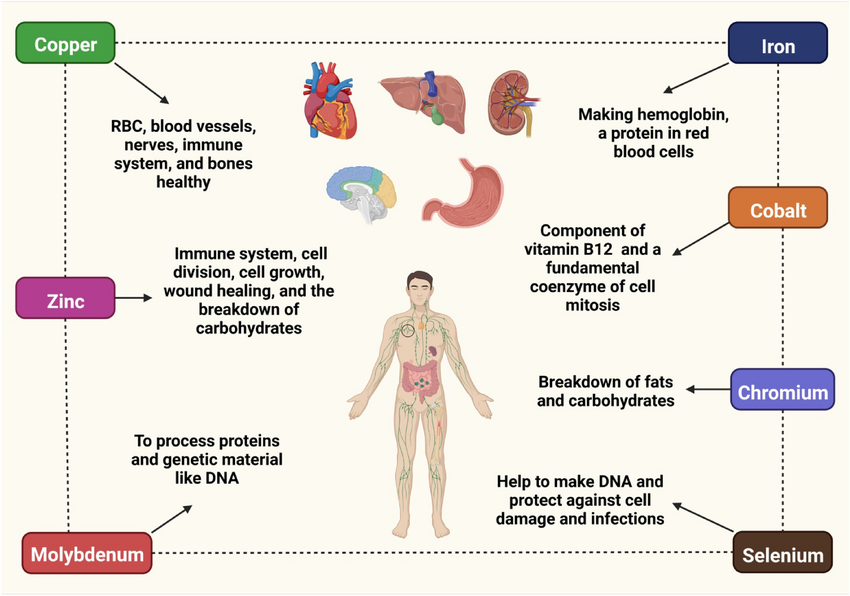
➤ Copper: This is part of body tissues, like the liver, brain, kidneys and heart.
➤ Fluorine: This is part of the teeth.
➤ Phosphorus: Helps with protein formation
➤ Iron: This integrates hemoglobin. In addition, it is involved in cellular respiration, glucose oxidation, fatty acid oxidation and DNA synthesis.
➤ Manganese: It is a part of certain enzymes. If you do not have enough, it can cause weight loss, dermatitis and nausea. Additionally, it may play a role in sexual and reproductive functions.
➤ Magnesium: This helps with glucose metabolism.
➤ Potassium: It balances the internal environment.
➤ Sodium: Like potassium, this also balances the internal environment.
➤ Iodine: This is necessary for thyroid function.
➤ Zinc: It is involved in the metabolism of proteins and nucleic acids. Therefore, it plays a very important role in pregnancy and fetal development. In addition, it promotes the activity of many enzymes.
Sources of Trace Elements:
Iron
- Liver, meat, poultry, fish, cereals, green leafy vegetables, legumes, peas, beans, nuts, oilseeds, jaggery and dried fruits.
Iodine
- Drinking water contains 0.5 mg/litre of fluorine.
- Foods Sea fish, cheese, tea etc
Zinc
- Liver, beef, lamb, other meats, whole meal wheat, flour, bread, nuts, legumes.
Copper
- Common salt, banana, tomatoes, Green leafy vegetables.
IRON
Iron (Fe):
Iron is of great importance in human nutrition. The adult human body contains between 4 to 5 gm of iron, of which about 60-70 percent is present in the blood (Hb iron) as circulating iron, and the rest (1 to 15 gm) as storage iron. Each gram of hemoglobin contains about 3.34 mg of iron. Unhealthy human beings the iron reserve is 1000 mg but in menstruating women in the plasma bound to a beta-globulin, transferring also known as siderophilin. Absorption of iron takes place in ferrous forms. Absorption of iron takes in the duodenum and upper jejunum. Fresh vegetables and green leafy vegetable contains iron. Deficiency of iron causes anaemia.
(Ref: Onila Salin’s Essential nutrition/1/43)
Sources of iron: There are two forms of iron –
1. Animal sources (Haem iron):
- Liver
- Meat.
- Poultry.
- Fish.
- Kidney.
- Heart.
- Egg Yolk
2. Plant or vegetable sources (Non-haem iron):
- Cereals
- Green leafy vegetables.
- Legumes
- Peas
- Beans
- Nuts
- Oilseeds
- Jaggery and
- Dried fruits.

Functions of Iron:
1. Formation of haemoglobin.
2. Brain development and function.
3. Regulation of body temperature.
4. Muscle activity and catecholamine metabolism.
5. Oxygen transport” and cell respiration are the main function of iron.
6. A lack of iron directly affects the immune system. It diminishes the number of T-cells and the production of antibodies.
7. Iron is essential for binding oxygen to the blood cells.
8. Iron is required as co-factor for other enzymes
(Ref: T. K. Indrani/1″/56)
Routes of iron loss:
- Physiological: menstruation, child birth
- Pathological: hook worm infestation, malaria, bleeding peptic ulcer, bleeding hemorrhoids
- Basal loss: Loss through urine, sweat and bile.
Total daily loss of iron:
- In adult: 1 mg
- Menstruating female: 2 mg
Effects of iron deficiency:
1. Nutritional anaemia (iron deficiency anaemia).
2. Impaired cell mediated immunity.
3. Reduced resistance to infection.
4. Increase morbidity and mortality
5. Diminished work performance.

Daily requirement of iron in different age:
| Age group | Requirement |
| Infants and children | 20-25 mg |
| Adolescent | 20-35 mg |
| Adults male | 24 mg |
| Adults female | 32 mg |
| Pregnancy | 40 mg |
| Lactating mother | 32 mg |
Causes of iron deficiency in our country:
1. Inadequate availability of iron (Fe) from the diet.
2. Increased blood loss.
3. Increased iron requirements.

Read More….