Covalent Bonding – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Covalent Bonding
Covalent bonding is the sharing of electrons between atoms. This type of bonding occurs between two atoms of the same element or of elements close to each other in the periodic table. This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals.
If atoms have similar electro-negativities (the same affinity for electrons), covalent bonds are most likely to occur. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable. In addition, the ionization energy of the atom is too large and the electron affinity of the atom is too small for ionic bonding to occur.
For example: carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. To form ionic bonds, Carbon molecules must either gain or lose 4 electrons. This is highly unfavorable; therefore, carbon molecules share their 4 valence electrons through single, double, and triple bonds so that each atom can achieve noble gas configurations. Covalent bonds include interactions of the sigma and pi orbitals; therefore, covalent bonds lead to formation of single, double, triple, and quadruple bonds.
Example: PCl3
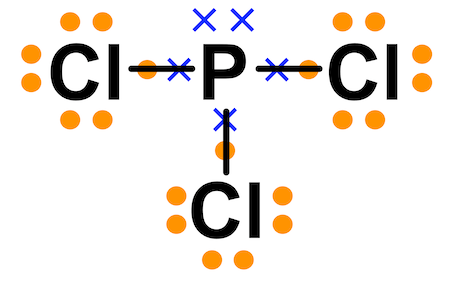
In this example, a phosphorous atom is sharing its three unpaired electrons with three chlorine atoms. In the end product, all four of these molecules have 8 valence electrons and satisfy the octet rule.

Definition of Covalent Bonds:
A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
or
A covalent bond in chemistry is a chemical link between two atoms or ions in which the electron pairs are shared between them. A covalent bond may also be termed a molecular bond.
Difference between Ionic Bonds and Covalent Bonds
| Traits | Ionic Bonds | Covalent Bonds |
| Description | Bond between metal and nonmetal. The nonmetal attracts the electron, so it’s like the metal donates its electron to it. | Bond between two nonmetals with similar electro-negativities. Atoms share electrons in their outer orbitals |
| Polarity | High | Low |
| Shape | No definite shape | Definite shape |
| Melting Point | High | Low |
| Boiling Point | High | Low |
| State at Room Temperature | Solid | Liquid or Gas |
| Examples | Sodium chloride (NaCl), Sulfuric Acid (H2SO4) | Methane (CH4), Hydrochloric acid (HCI) |
| Chemical Species | Metal and nonmetal (remember hydrogen can act either way) | Two nonmetals |
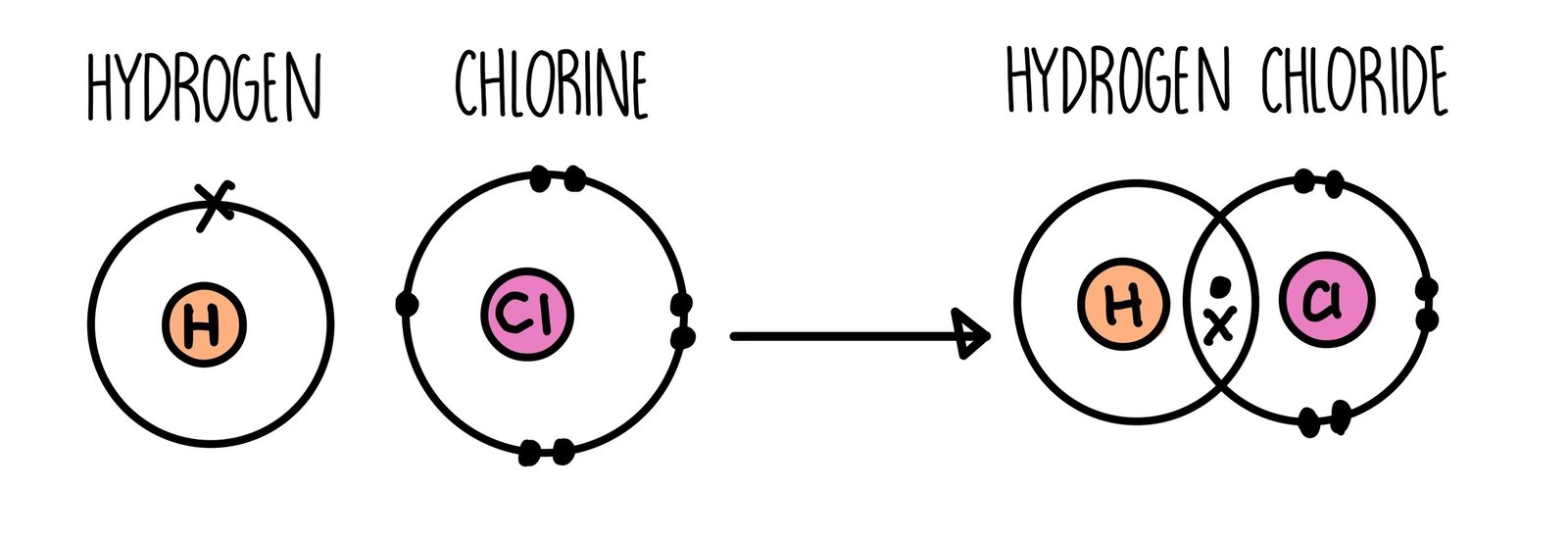
covalent bond
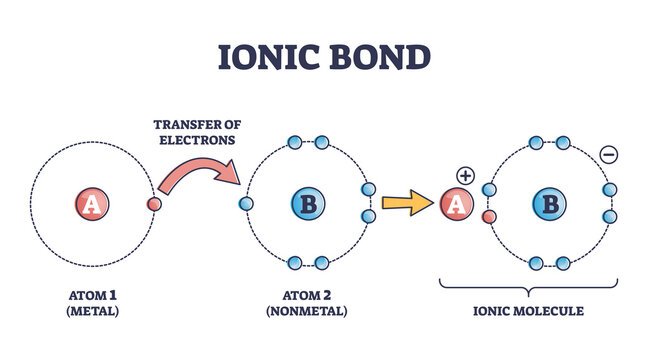
ionic bond
Read More….