Osmosis, Osmotic Pressure and Osmolarity – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Osmosis, Osmotic Pressure and Osmolarity
Osmosis:
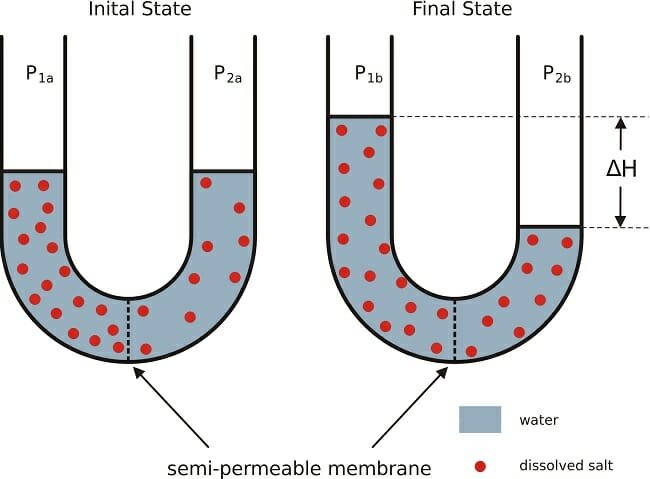
The migration of solvent molecules from the region of lower solute concentration to the region of higher solute concentration across a semipermeable membrane is called osmosis.
Condition of osmosis:
- Two solution of different osmotic pressure.
- Presence of a semipermeable membrane.
Importance of osmosis:
A. Physiological:
- Absorption from GIT.
- Fluid exchange in the capillary bed.
- Regulation of urine formation.
- Reabsorption of CSF.
- Maintenance of Blood pressure
B. Clinical:
- Infusion: Isotonic solutions of NaCl (0.9%) or glucose (5%) are commonly used as infusion in hospitals for the treatment of dehydration, burn etc.
- Action of Purgatives: Purgatives (e.g. MgSO4) prevent water absorption by osmosis from GIT. Thus they cause dilution of intestinal content and helps in defecation.
- Osmotic diuresis: The high blood glucose concentration (e.g. in Diabetes mellitus) causes osmotic diuresis, resulting in the loss of water, electrolytes and glucose in the urine.
- Edema due to hypoalbuminaemia: When plasma protein is decreased, water leaves blood vessels and enter into the interstitial space by osmosis, thus edema develops.
- Cerebral edema: In cerebral edema, hypertonic solution is used that causes osmosis of water from brain and thus prevents edema.
- Irrigation of wounds: Isotonic solutions are used for washing wounds. The pain experienced by the direct addition of salt or sugar to the wounds is due to osmotic removal of water.
Definition of Osmotic Pressure:
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in pure solvent (which belongs to the solution under discussion) by osmosis.
or
The amount of pressure required to prevent osmosis completely is called osmotic pressure. Osmotic pressure causes fluid movement by osmosis from the interstitial space into the blood. It is measured as Osmole/L or milliosmole/L. The plasma colloidal osmotic pressure is 280-300 mOsm/L.
Haemodialysis
Definition of Haemodialysis
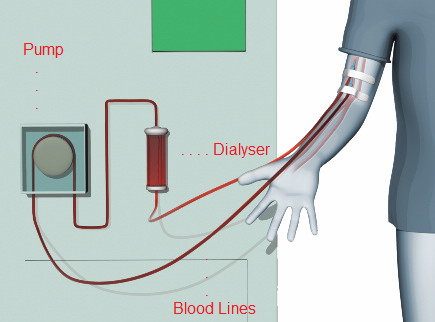
Hemodialysis, also spelled haemodialysis, commonly called kidney dialysis or simply dialysis, is a process of purifying the blood of a person whose kidneys are not working normally. This type of dialysis achieves the extracorporeal removal of waste products such as creatinine and urea and free water from the blood when the kidneys are in a state of kidney failure.
Indication of Haemodialysis
Indications of dialysis in acute renal failure (ARF)
- Severe fluid overload
- Refractory hypertension
- Uncontrollable hyperkalemia
- Nausea, vomiting, poor appetite, gastritis with hemorrhage
- Lethargy, malaise, somnolence, stupor, coma, delirium, asterixis, tremor, seizures,
- Pericarditis (risk of hemorrhage or tamponade)
- Bleeding diathesis (epistaxis, gastrointestinal (GI) bleeding and etc.)
- Severe metabolic acidosis
- Blood urea nitrogen (BUN)> 70-100 mg/dl
Indications of dialysis in chronic renal failure (CRF)
- Pericarditis
- Fluid overload or pulmonary edema refractory to diuretics
- Accelerated hypertension poorly responsive to antihypertensives
- Progressive uremic encephalopathy or neuropathy such as confusion, asterixis, myoclonus, wrist or foot drop, seizures
- Bleeding diathesis attributable to uremia

Risks Associated With Hemodialysis
Hemodialysis risks include:
- Low blood pressure
- Anemia, or not having enough red blood cells
- Muscle cramping
- Difficulty sleeping
- Itching
- High blood potassium levels
- Pericarditis, an inflammation of the membrane around the heart
- Sepsis
- Bacteremia, or a bloodstream infection
- Irregular heartbeat
- Sudden cardiac death, the leading cause of death in people undergoing dialysis

Read More….