The artificial kidney: A hemodialysis machine – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
The artificial kidney: A hemodialysis machine
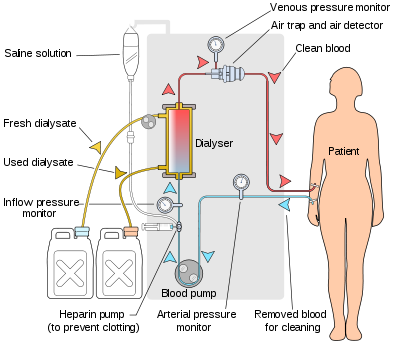
Dialysis Machine
A machine used in dialysis that filters a patient’s blood to remove excess water and waste products when the kidneys are damaged, dysfunctional, or missing. The dialysis machine itself can be thought of as an artificial kidney.
Inside, it consists of more plastic tubing that carries the removed blood to the dialyser, a bundle of hollow fibers that forms a semipermeable membrane for filtering out impurities. In the dialyser, blood is diffused with a saline solution called dialysate, and the dialysate is in turn diffused with blood. When the filtration process is complete, the cleansed blood is returned to the patient.
Most patients who undergo dialysis because of kidney impairment or failure use a dialysis machine at a dialysis clinic. Also, a machine called a peritoneal dialysis machine can be used chronically at home for dialysis, which eliminates the need for regular hemodialysis clinic treatments. Using this machine during the day and frequently during sleep, the patient can control his/her own dialysis.
Definition of Osmolarity:
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L).
Necessity of Osmotic Pressure:
1. The food is digested and substances pass into the blood by osmosis.
2. While administering drugs intravenously, drugs should be dissolved in physiological saline solution because the tissues are not changed by osmosis.
3. In hypotonic saline solution, the living red corpuscles will begin to swell and finally rupture because water passes in through the cell wall by osmosis to dilute the more concentrated cell contents. This disintegration of red corpuscles is called as hemolysis. It causes death of the patient.
4. In a hypertonic salt solution the water flows out from the corpuscles more rapidly than it flows in, and the cells become shrink. This process is called crenation or plasmolysis.
5. In certain pathological conditions hypertonic solution, of salt is given, e.g., in disease accompanied by oedema, a concentrated solution of magnesium sulphate is given by mouth. Because, the high concentration of salt withdraws the fituid from the tissues into the intestine and eliminates it through the intestinal tract.
Colloidal osmotic pressure (COM):
The osmotic pressure that is exerted by the colloids is called colloidal osmotic pressure. Plasma colloidal osmotic pressure is the osmotic pressure exerted by the plasma proteins. COM causes fluid movement from the interstitial space to the blood by osmosis.
Importance:
- COM prevents ents loss of fluid from the blood into the interstitial space.
- COM causes osmosis of fluid inward through the capillary membrane. Thus it prevents formation of edema
- It controls the intracellular fluid volume as well as interstitial fluid volume.
- It plays an important role in the regulation of the glomerular filtration.
- ↓COM results in the development of edema.
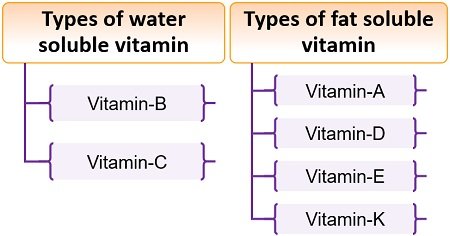
Solubility of Vitamins
A. Fat-soluble vitamins: Fat-soluble vitamins are stored in the fatty tissues of the body and the liver. Fat-soluble vitamins are easier to store than water-soluble ones and can stay in the body as reserves for days, some of them for months. Fat-soluble vitamins are absorbed through the intestinal tract with the help of fats (lipids).
Example: Vitamins A, D, E, and K are fat-soluble.
B. Water-soluble vitamins: Water-soluble vitamins do not get stored in the body for long – they soon get excreted in urine. Because of this, water-soluble vitamins need to be replaced more often than fat-soluble ones.
Example: Vitamins C and all the B vitamins are water-soluble.
NICE TO KNOW
Classifications of Vitamins According to Their Solubility:
1. Fat soluble vitamins:
- Vitamin A or retinol.
- Vitamin D.
- Vitamin E.
- Vitamin K.
2. Water soluble vitamins:
- Vitamin B complex:
✓ Thiamine (vitamin B1)
✓ Riboflavin (vitamin B2)
✓ Nicotinic acid.
✓ Biotin.
✓ Pyridoxine (vitamin B6)
✓ Pantothenic acid(vitamin B5)
✓ Folic acid.
✓ Lipoic acid.
✓ Vitamin B12.
- Ascorbic (vitamin C)
| Name | General Reaction Pattern |
| Combination or synthesis | A+B ->AB |
| Decomposition | AB-> A + B |
| Substitution or Single Replacement | AB->CB + AC |
| Metathesis or Double Displacement | AB + CD-> AD + CB |
Combination or Synthesis Reaction:
A combination or synthesis reaction is one, where a new product is synthesized by combination of two or three reactants.
Example
- Hydrogen + Oxygen →→ Water
- H2+O2→→ H2O
In this reaction, hydrogen and Oxygen combine to form water. So, since they are combining to form a new product, and a new compound, water is synthesized here, this reaction is said to be a synthesis reaction.
Decomposition Reaction
Decomposition reaction is one, where one compound decomposes or breaks into two or more different products.
Example
- Lead nitrate→→ Lead monoxide + Nitrogen dioxide + Oxygen
- Pb(NO3)2 PbO + NO2 + O2
Displacement or Replacement Reaction
There are two types of displacement reaction.
1. Single displacement reaction: When a cation or an anion is exchanged from a compound, this is called as single displacement reaction.
- Example: Zn + H2SO4→→ ZnSO4 + H2
In the above reaction, zinc replaces hydrogen from hydrogen sulphate or sulfuric acid, to form zinc sulfate. Since only cation is exchanged here, this is a single displacement reaction
2. Double displacement reaction: The anions are exchanged between two compounds, or salts. Such reactions results in different combination of cations and anions, at the end.
- Example: BaCl2 + Na2SO4→→ BaSO4 + 2NaCI
Chloride ion leaves Barium and gets attached itself to sodium. In this process, sulfate ion leaves sodium and attaches itself to Barium. Thus, there is an exchange of anions among Barium and sodium resulting in a double replacement or displacement reaction. Since both the compounds are changing, it is different from a single displacement reaction.
or
Acid Base Reactions
An acid and a base combines to give salt and water. This reaction is called as a neutralization reaction or just acid-base reaction.
Example
- HBr + KOH→→ H2O + KBr
Combustion Reaction
A reaction where mostly an organic compound burns in the presence of oxygen to yield mostly carbon dioxide, water, and other products, is also a type of combination reaction. Combination of any substance with oxygen results in combustion, leading to the burning of the compounds to its elementary products.
Example
- C4H10O2→→ CO2 + H₂O

Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time.
The chemical equilibrium is achieved when the rate of forward reaction is same as the reverse reaction. Since the rates are equal, there are no net changes in the concentrations of the reactant(s) and product(s). This state is known as dynamic equilibrium.

Read More….