Concept about Oxygen – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Concept about Oxygen
Oxygen is a chemical element with symbol O and atomic number 8.
It is a member of the chalcogen group on the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. By mass, oxygen is the third-most abundant element in the universe, after hydrogen and helium.
At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula O2. Diatomic oxygen gas constitutes 20.8% of the Earth’s atmosphere. As compounds including oxides, the element makes up almost half of the Earth’s crust.
Definition of Oxygen:
Oxygen is the odorless gas that is present in the air and necessary to maintain life. Oxygen may be given in a medical setting, either to reduce the volume of other gases in the blood or as a vehicle for delivering anesthetics in gas form.
or
Oxygen is a colorless and odorless gas which is essential for life. Normally a person will take the oxygen required by the body, normal breath and taken up into the blood.
Preparation Process of 02:
1. Oxygen is usually prepared commercially from air, which essentially a mixture of this element and nitrogen. Air is liquefied by subjecting it to a high pressure at a low temperature. When the liquid air is allowed to evaporate, the more volatile nitrogen escapes first, leaving behind the pure oxygen.
Pure oxygen is also obtained for commercial purposes by the electrolysis of water. When the electric current is passed through water, oxygen forms at the anode, and hydrogen forms at the cathode
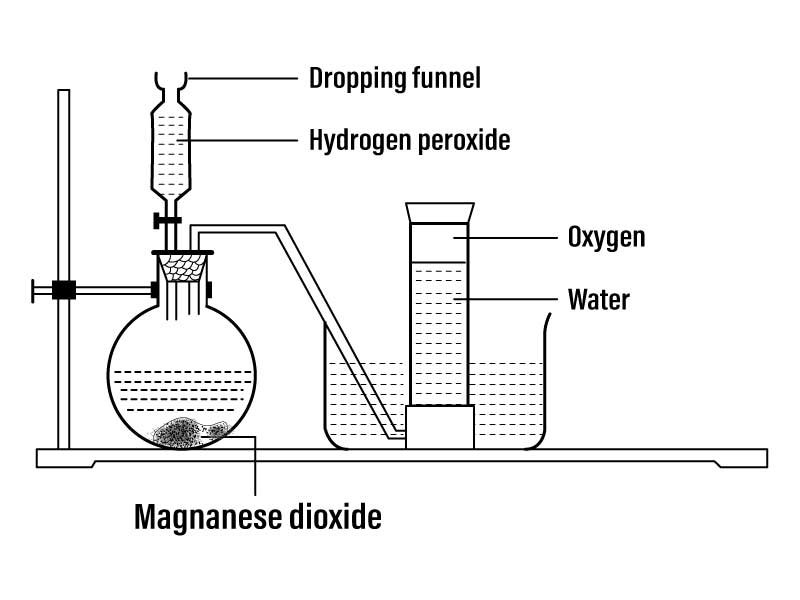
2. In the laboratory, oxygen is usually prepared by heating potassium chlorate (KC103) in the presence of a catalyst, i.e., manganese dioxide (MnO2). A substance of this kind that can influence the speed of a chemical reaction without being altered itself is called catalyst. The chemical reaction is as follows:
- 2KClO3 + (MnO2) -> Heat -> 2KC1+3O2↑
Physical Properties:
1. Oxygen is a colourless, odourless, and tasteless gas,
2. It is slightly heavier than air, and 16 times heavier than hydrogen.
3. It is liquefied at a high pressure (50 atmospheres) and at a low temperature of -118°C.
4. Oxygen is slightly soluble in water, i.e., 3c.c. of oxygen will dissolve in, 100 c.c. of water at ordinary temperature
Chemical Properties:
1. Oxygen does not burn itself but it supports burning
2. It is very active at high temperature. The union of substance with oxygen is called oxidation, i.e., an increase in the valence number of an element in a reaction.
3. Most of the organic substances are oxidized to carbon dioxide, water, and ashes.
4. In the rusting of iron, the decay of plant and animal matter, and oxygenation of food
materials in the body is a slow process of oxidation.
5. Formation of oxides:-all the elements combine with oxygen form oxides such as iron oxide (rust), silicon dioxide (sand), etc
6. Oxidizing agent:-An oxidizing agent is one which gives up oxygen readily to another substance, e.g., Hydrogen peroxide, H2O2; Potassium permanganate, KMnO4. These substances are unstable and decompose readily when exposed to sunlight, heat, and air. Hence, they should be kept in dark coloured bottles with tight stoppers and kept in a cool place. Oxidizing agents are used as bacteria killers e.g., KMnO4. They have a bleaching effect, e.g., H₂O
Uses of Oxygen
1. All forms of animal life need oxygen to live. Animals living in land, they get oxygen from the air. Aquatic animals get oxygen from the air dissolved in water.
2. When oxygen is breathed into the lungs, it diffuses into the blood and loosely combines with hemoglobin (red pigment of the blood, commonly RBCs). This unstable compound of oxygen and hemoglobin is called oxy-hemoglobin, which readily gives up oxygen to the tissue cells.
3. Oxygen circulates through tissues to all parts of the body oxidizing the sugars, fats all broken down tissues and certain poisonous substances with the formation of heat, lactic acid, CO₂ and water. The water is excreted by kidneys, skin, and lungs. Whereas CO2 is carried by the blood to the lungs and exhaled.
4. Oxygen decomposes the refuse organic matter by a process called decay.
5. Oxygen is used to revive cases of drowning, Carbon monoxide, CO poisoning, asphyxiated new-born babies, i.e., who experience difficulty in breathing, patients suffering from pneumonia and other lung diseases.
6. Oxygen is used by aviators, sailors, and workers engaged in mines and fire rescue work.
7. An important industrial use of oxygen is in the production of intense heat for cutting and welding iron and steel plates. When Oz is mixed with H₂ or acetylene, C₂H₂ gas and the mixture is ignited, the very hot flame produced is sufficient to melt steel, i.e., oxyacetylene flame.
Definition of Oxygen Therapy:
Oxygen therapy refers to the administration of supplemental oxygen as part of managing illness. In healthy individuals, oxygen is absorbed from the air in adequate amounts, but certain diseases and conditions can prevent some people from absorbing enough oxygen.
or
Oxygen therapy is the administration of oxygen as a medical intervention, which can be for a variety of purposes in both chronic and acute patient care.
Purposes of Oxygen Therapy:
1. To relieve dyspnea
2. To improve tissue oxygenation
3. Decreased work of breathing (WOB) in dysenteric clients.
4. Decreased work of the heart in clients with cardiac disease.
5. To administer low/higher concentration of oxygen to patients,
6. To allow uninterrupted supply of oxygen during activities like eating, drinking etc.

Factors Affecting Oxygenation:
Adequate of circulation, ventilation, perfusion and transport respiratory gases to the tissues are influenced by four types of factor:
A. Physiological factors:
Any condition that affects cardiopulmonary functioning directly affects the body’s ability to meet oxygen demand
B. Developmental factor:
The developmental stage of the client and the normal aging process can affect tissue oxygenation, e.g. children are at risk of acute upper respiratory tract infections and exposure to these infections.
C. Behavioral factors:
A person’s behavior or lifestyle may directly or indirectly affect the body’s ability to meet oxygen requirements. Lifestyle factors that influence respiratory functioning include, nutrition (e.g. obesity and malnutrition), exercise (lack of exercise), cigarette smoking, substance abuse (excessive alcohol, drug addiction), and stress
(severe anxiety).
D. Environmental factor:
The environment can also influence oxygenation. The incidence of pulmonary disease is higher in smoggy, urban areas than rural areas. In addition client’s workplace may increase the risk for pulmonary disease. Occupational pollutions include asbestos, talcum powder, dust and airborne fibers leads to occupation diseases.
Complications of Oxygen Therapy:
100% oxygen is both irritant and toxic if inhaled for more than few hours.
- Premature infants develop retrolental fibroplasia and blindness if exposed to excessive concentrations.
- In adults, pulmonary oxygen toxicity (manifested by pulmonary oedema and free radical damage leading ultimately to fibrosis.

Indications of Oxygenation:
A. Any individual with one or more of the following:
1. Peri and post cardiac or respiratory arrest
2. Hypoxia diminished blood oxygen levels (oxygen saturation levels of <92%
3. Acute and chronic hypoxemia
4. Signs and symptoms of shock
5. Low cardiac output and metabolic acidosis (HCO3 18mmol/l)
6. Chronic type two respiratory failure (hypoxia and hypercapnia
B. Despite a lack of supportive data, oxygen is also administered in the following conditions:
1. Dyspnoea without hypoxemia
2. Post-operatively, dependent on instruction from surgical team
3. Treatment of pneumothorax
4. Anaemia
5. Asphyxiai
6. Poisoning
7. Drowning
8. During severe case of injury
9. Bronchial asthma
Contraindications of Oxygen Therapy:
| Relative Contraindications | Reason Contraindicated |
| Pacemakers or epidural pain pump |
|
| Pregnancy |
|
| Seizures |
|
| Upper respiratory infection (URI) |
|

Read More….