Concept about Salt – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Concept about Salt
Definition of Salts:
Salts are ionic compounds whose cations, i.e., positive ions are any except H¹ and whose anions (negative ions) are any except OH. All salts are crystalline solids at room temperature.
or
The word “salt” is a general chemical term that refers to ionic compounds formed when an acid reacts with a base. They may be simple salts such as NaCl, KCI, and Na 2SO 4; acid salts like NaHCO 3 and NaH 2 PO 4

Formation of Salts:
Salts are formed by a chemical reaction between:
- A base and an acid, e.g., NH3 + HCI → NH4CI
- A metal and an acid, e.g., Mg + H2SO4 → MgSO4 + H2
- A metal and a non-metal, e.g., Ca + Cl2 → CaCl₂
- A base and an acid anhydride, e.g., 2 NaOH + Cl₂O 2 NaCIO + H₂O
- An acid and a base anhydride, e.g., 2 HNO3 + Na2O2 NaNO3 + H2O
- Salts can also form if solutions of different salts are mixed, their ions recombine, and the new salt is insoluble and precipitates (see: solubility equilibrium), for example: Pb(NO3)2 + Na2SO4→ PbSO4↓ + 2 NaNO3
Importance of Salts in Human Body
1. Acids are constantly being formed in the body. The body fluids are either alkaline or neutral except the gastric juices and urine. As a result of neutralization, many salts are produced and excreted in urine sweat, and faces.
2. Any change in the normal acidity or alkalinity of the different parts of the body effects every cell and organ, e.g., if the alkalinity of the blood is too low (i.e., acidosis) or too high (i.e., alkalosis), the heart may be affected so that it will not pump uniformly.
3. The process of digestion and cell activity produce numerous substances which enter in blood stream. These substances may be acidic, alkaline, and other neutral. The blood maintains a constant concentration of H+ that ranges from 7.3 to 7.5 whenever the pH falls within the range of slightly less than 7.3 to 7.0, a person is said to have acidosis caused by an increase in hydrogen ion concentration. Alkalosis occurs within the range 7.5 to 7.8 due to decrease in pH value.
4. Body fluids contain three major buffer systems, (i.e., shock absorbers). First, bicarbonate ion, HCO 3, second is bi phosphate ion, H2PO4, and the third major buffer is proteins. Buffer systems maintain the constancy of pH in the blood to avoid acidosis and alkalosis
Acidosis:
When the pH of the body goes below 7.35 (lower limit of normal range), then it is called acidosis.
(Normal pH of body fluid: 7.35-7.45, average: 7.4)
Classification:
It is of two types-
➤ Metabolic acidosis.
➤ Respiratory acidosis.
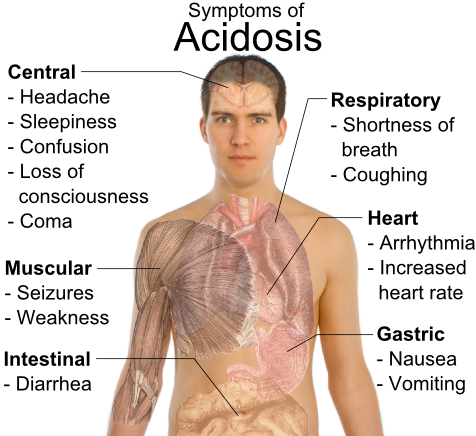
Effects of acidosis:
The major effect of acidosis is depression of the CNS. The nervous system become so depressed that the person becomes disoriented and later comatose.
Therefore patient may die due to diabetic acidosis, uramic acidosis etc.
Effects of alkalosis:
The effects of alkalosis are the over excitation of the nervous system. The muscles may go into a state of tetany i.e. a state of tonic spasm.
Extremely alkaline patient may die due to tetany of the respiratory muscles.
Simple acid base disorders:
If the pH change occurs due to change of either HCO3 or PCO2, this is called simple acid base disorders.
Classification: simple acid base disorders can be classified into four groups-
➤ Metabolic acidosis
➤ Metabolic alkalosis
➤ Respiratory acidosis
➤ Respiratory alkalosis.
Systems that concerned with acid-base balance:
1. Buffer system: works within seconds. Buffers combine with acid or base to prevent any change of PH
2. Respiratory system: works within minutes to hours. It regulates the removal of CO2 from the ECF.
3. Renal system: works within hours to days. It regulates the ECF H+ by excreting acidic or alkaline.
➤ 1st line defense: Buffer system.
➤ 2nd line defense: Respiratory system.
➤ 3rd line defense: Renal system. [it is the most powerful acid-base regulatory system.]
Metabolic acidosis:
Decreased pH due to reduced plasma HCO concentration is called metabolic acidosis.
➤ Primary defect: plasma HCO3 due to any cause.
➤ Mechanism: plasma HCO→↑ H→ pH metabolic acidosis.
➤ Clinical causes of metabolic acidosis: [either due to increased production or decreased excretion of acids.]
- Severe diarrhea (Loss of HCO3-).
- Diabetic ketoacidosis.
- Renal failure.
- Lactic acidosis.
- Prolonged starvation.
NOTE:
Causes of metabolic acidosis:
- Increased production of metabolic acid. e.g- Diabetic ketoacidosis, Lactic acidosis, Fever (Here↑ rate of normal metabolism occur).
- Decreased excretion of metabolic acids (due to renal failure).
- Increased loss of base/alkali from the body. (e.g- In diarrhoea).
- Administration of acidic drug. (e.g- Paracetamol, etc)
Respiratory system (Primary compensation):
By increasing respiratory rate and thereby Pco2.
Mechanism: pH →↑ H¹ respiratory center→↑ respiration (hyperventilation) → ↑ CO₂ elimination→ PCO2→↑ pH pH backs towards normal → Acidosis is corrected.
Renal compensation (Secondary compensation):
By adding new HCO3 in the plasma and thereby ↑ pH.
Mechanism: pH↑ H+ ↑ H+ secretion into the renal tubules reabsorption of all filtered HCO3- &
↑ generation of new HCO3→↑ plasma HCO3→↑ pH pH backs towards normal →→ Acidosis is corrected.
(Ref: Guyton and Hall 12th 391p)
Comparison between metabolic & respiratory acidosis:
| Metabolic acidosis | Respiratory acidosis |
| pH due to primary alkali (HCO3) deficit is called metabolic acidosis. | ↑ arterial PCO2 leads to fall of pH is called respiratory acidosis. |
| Due to accumulation of fixed acid. | Due to accumulation of volatile acid. |
| Plasma HCO3- & PCO2. | ↑ Plasma HCO3 & PCO2 |
Causes:
| Causes:
|
Compensation: Compensated by:
Body fluid buffer system | Compensation: Compensated by renal system. |
Why patient of diabetic ketoacidosis suffer from metabolic acidosis?
Patients of diabetic ketoacidosis suffer from metabolic acidosis because, in diabetic ketoacidosis there is increased amount of ketone bodies in the blood all of which are relatively strong acids causing acidosis (metabolic acidosis). Both acetoacetate & ẞ-hydroxybutyrate are strong acids.
Respiratory acidosis:
Increased arterial PCO2 due to hypoventilation which leads to fall of pH, is called respiratory acidosis.
➤ Primary defect: ↑ arterial Pco, due to any cause.
➤ Mechanism: Hypoventilation →↓ CO2 elimination→↑ arterial Pco₂→↓ pH → Respiratory acidosis.
➤ Causes of Respiratory acidosis:
- Chronic obstructive pulmonary disease (COPD).
✔Bronchial asthma.
✔ Emphysema of the lung. - Fibrosis of the lungs.
- Hyaline membrane disease.
- Depression of the respiratory center by any disease or drugs.
- Acute pulmonary edema, Pneumonia.
➤ Compensation: It is compensated by-
- Renal system (Primary compensation).
- Respiratory compensation (Secondary).
➤ Renal compensation: By ↑ in plasma HCO3 by addition of new HCO3 to the ECF.
➤ Mechanism: ↑ Pco₂→↑ H2CO3 production by the kidneys→↑ H’ (from H2CO3)→↑ H secretion in the renal tubules complete reabsorption of filtered HCO &↑ generation of new HCO3→↑ plasma HCO3→↑ pH pH backs toward normal.
➤ Respiratory compensation: By developing hyperventilation. pH &↑ Pco2 stimulates the respiratory center and thereby causes hyperventilation.
((Ref: Guyton and Hall 13th 391)
Lactic acidosis:
Increased concentration of lactic acid (lactate) in the plasma is known as lactic acidosis. It may occur due to its increased production or decreased utilization.
Causes:
1. Mild form (not life-threatening):
➤ Heavy exercise.
➤ Respiratory diseases.
➤ Shock.
➤ Cancer etc.
2. Severe form (life threatening): It occurs due to impairment/ collapse of the circulatory system in-
➤ Myocardial infarction (MI).
➤ Uncontrolled hemorrhage.
➤ Severe shock.
➤ Pulmonary embolism etc.
Alkalosis:
When the pH of the body increases above 7.45 (upper limit of normal range of pH) due to accumulation of bases or loss of acids from the body, then it is called Alkalosis.
Classification: It is of two types-
➤ Metabolic alkalosis: Alkalosis in which plasma HCO3- is increased and a decrease in plasma H is called metabolic alkalosis.
➤ Respiratory alkalosis: Alkalosis with an acute reduction of plasma Pco2 resulting in increased pH is called respiratory alkalosis.
Respiratory alkalosis:
Respiratory alkalosis rarely found than respiratory acidosis.
➤ Primary defect: arerial Pcoz which is caused by hyperventilation due to any cause.
➤ Mechanism: Hyperventilation →↑ CO2 elimination →↓ arterial Pco₂→↑ pH → Respiratory alkalosis.
➤ Causes of respiratory alkalosis: (any causes of hyperventilation)
- Hyperventilation due to high altitude or hypoxia.
- Lobar pneumonia, High fever.
- Hysteric hyperventilation
- Over dose of cardio-respiratory stimulating drugs e.g. salicylate poisoning.
- Hepatic failure.
➤ Compensation:
Renal compensation (Primary compensation): By plasma HCO3 by ↑ renal excretion of HCO3.
Mechanism: Pco₂H₂CO3 production in the renal tubular cells→ H+H secretion into the renal tubule →↓ reabsorption of filtered HCO3 & no generation of new HCO3→↓ plasma HCO3 →↓ pH pH backs towards normal → Alkalosis is corrected.
Respiratory compensation (Secondary compensation): By producing hypoventilation. ↑ pH & Pco2 causes hypoventilation and ↑ H2CO3
(Ref: Guyton and Hall/ 12th /393)
Metabolic alkalosis:
➤ Primary defect: ↑ plasma HCO3 due to any cause.
➤ Mechanism: ↑ plasma HCO3 →↑ pH →metabolic alkalosis.
➤ Causes of metabolic alkalosis:
- Severe vomiting (containing gastric contents only).
- Iatrogenic: Prolong diuretics therapy.
- Excessive ingestions/administration of alkaline drugs (e.g. NaHCO3) and foods.
- Excess aldosterone secretion.
- Massive blood transfusion containing citrate.
Compensation:
➤ Respiratory system (Primary compensation): By developing hypoventilation and thereby ↑ Pco2.
Mechanism:↑ pH →↑ H+ which inhibit the respiratory center →↓ respiration (hypoventilation) → CO2 elimination→↑ Pco2 pH pH backs toward normal
→ Alkalosis is corrected.
➤ Renal compensation (Secondary compensation): By increasing renal HCO3 excretion.
Mechanism:↑ PH→ H+ which causes H secretion into the renal tubules →→ reabsorption of filtered HCO3 & no generation of new HCO3 plasma HCO3→↓ pH pH backs toward normal Alkalosis is corrected.
Ref: Guyton and Hall 12th 393p.
NOTE:
Metabolic alkalosis is compensated by-
- Respiratory system: By Hypoventilation.
- Body fluid buffer system: It makes salt & water with alkali and brings it neutral. So alkalosis is decreased.
- Renal system: When there is alkalosis, it exerts more alkali (e.g-NaHCO3) and retains acids in the form of hydrogen ion.
NOTE:
Structures involved in lactic acid production are:
- Muscle
- Skin
- Brain
- RBC
When the term ‘respiratory’ is used, you should consider the pco2.
Say…
- Respiratory acidosis means → pco2
- Respiratory alkalosis means → pco2
When the term ‘metabolic’ is used, you should consider [HCO3].
Say…
- Metabolic acidosis means → HCO3
- Metabolic alkalosis means → HCO3
[Note: regarding compensation: when the term ‘respiratory’ is used, you should consider renal mechanism.
Say…
- Respiratory acidosis is compensated mainly by renal mechanism.
- Respiratory alkalosis compensated mainly by renal mechanism.
When the term ‘metabolic’ is used, you should consider respiratory mechanism.
Say…
- Metabolic acidosis is compensated mainly by respiratory mechanism.
- Metabolic alkalosis is compensated mainly by respiratory mechanism]
Laboratory features of acid base equilibrium disturbances.
| Plasma bicarbonate | Plasma PCO2 | Plasma pH | |
| Metabolic acidosis | ↓ | ↓ | ↓ |
| Respiratory acidosis | ↑ | ↑ | ↓ |
| Metabolic alkalosis | ↑ | ↑ | ↑ |
| Respiratory alkalosis | ↓ | ↓ | ↑ |

Why acidosis is common in chronic renal failure (CRF) and what is renal tubular acidosis?
Acidosis is common in CRF/ CKD because of failure to excrete the acids or acid products which are byproducts of digestion & metabolism.
Renal tubular acidosis:
It is the impairment of the ability of the kidney tubules to make the urine acidic although the other renal functions are usually normal. This type of acidosis results from a defect in renal secretion of H ^ + or in reabsorption of HCO3 or both.

Read More….
