Concept of Bioavailability – This book covers the entire syllabus of “Pharmacology” prescribed by BNMC- for diploma in nursing science & midwifery students. We tried to accommodate the latest information and topics. This book is an examination set up according to the teachers’ lectures and examination questions.
At the end of the book, previous questions are given. We hope in touch with the book students’ knowledge will be upgraded and flourish. The unique way of presentation may make your reading of the book a pleasurable experience.
Concept of Bioavailability
Bioavailability:
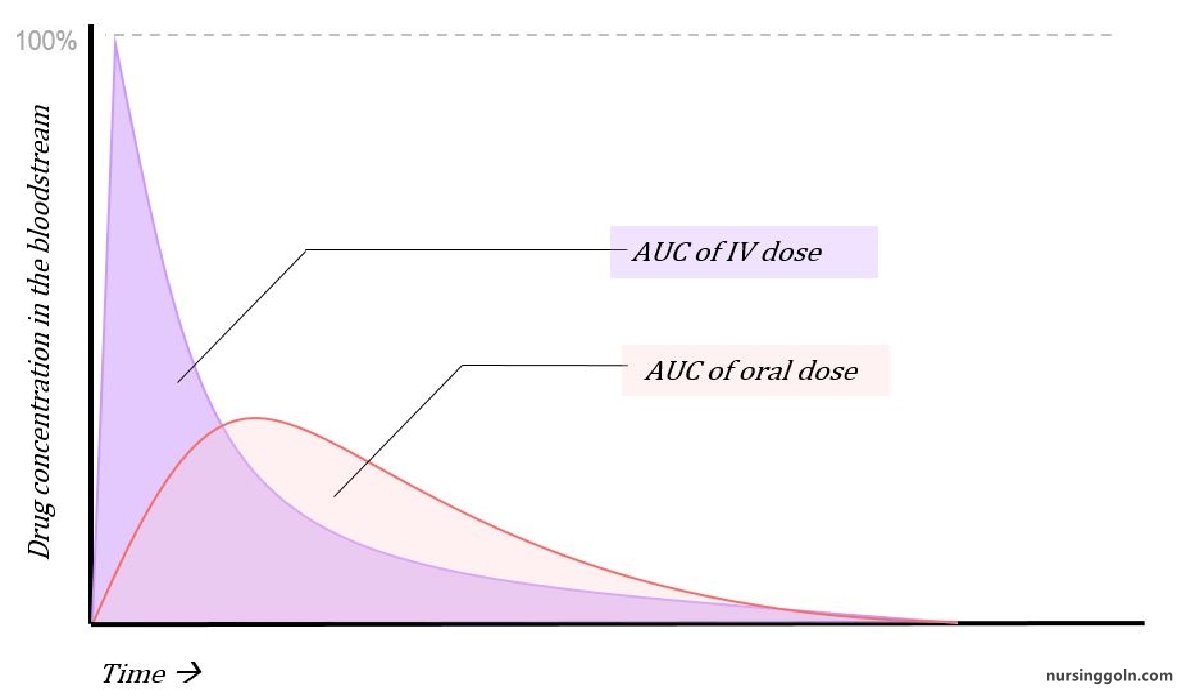
It is a measure of the fraction (F) of administered dose of a drug that reaches the systemic circulation in the unchanged form.
➤ Bioavailability of a drug is represented by %
➤ When a drug is given intravenously, its bioavailability becomes 100%
➤ If 100 mg of a drug is administered orally and 60 mg of this drug is absorbed unchanged, the bioavailability is 60%
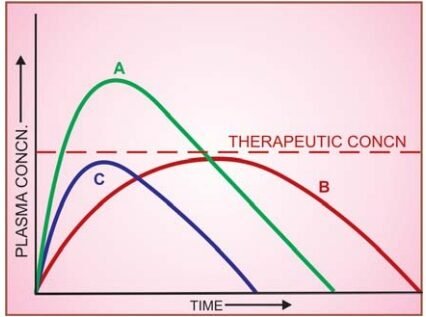
Fig: Plasma concentration-time curves depicting bioavailability differences between three preparations of a drug containing the same amount
Note that formulation B is more slowly absorbed than A, and though ultimately both are absorbed to the same extent (area under the curve same), B may not produce therapeutic effect; C is absorbed to a lesser extent-lower bioavailability
Rate: means onset and intensity of drug action.
Extent: means amount of drug absorbed & duration of action.
Significance of Bioavailability
➤ For comparing different formulation of same drug.
➤ For comparing different formulation of same drug produced by different companies.
Factors modifying bioavailability
➤ Pharmaceutical factors (factors of absorption).
- Formulation properties:
✔Disintegration time: more the disintegration time, less absorption and so less bioavailability.
✔Dissolution rate: more the dissolution rate more the bioavailability.
- Physical state of drug and particles size
✔Physical state: liquid > solid.
✔Size: crystalloid > colloid. More absorption more bioavailability
➤ Presystemic elimination (first pass metabolism).
➤ Enterohepatic circulation of drug.
➤ Biological factors.
Routes of administration, bioavailability and general characteristics
| Route | Bioavailability | Characteristics |
| Intravenous | 100% | More rapid onset |
| Subcutaneous | < 100% |
|
| Intramuscular | < 100% |
|
| Rectal | < 100% | Les-s first pass effect than oral |
| Transdermal | < 100% | Usually very slow absorption |
| Oral | < 100% |
|
| Inhalation | < 100% | Very rapid onset of action |
Bioequivalence:
Oral formulations of a drug from different manufacturers or different batches from the same manufacturer may have the same amount of the drug (chemically equivalent) but may not yield the same blood levels-biologically inequivalent. Two preparations of a drug are considered bioequivalent when the rate and extent of bioavailability of the active drug from them is not significantly different under suitable test conditions.
Half-life
Half-Life of a Drug
It is the time in which the concentration or effect of the drug declines by one half (half-life of drug is constant).
Half–life depends on
1. Route of administration.
2. Diffusion into tissue.
3. Plasma protein and tissue binding.
4. Metabolism of drug.
5. Excretion of drug.
Types of half-life
1. Plasma half-life
2. Biological or elimination half-life.
3. Biological effective half-life.

Plasma Half-Life
The Plasma half-life (t%) of a drug is the time taken for its plasma concentration to be reduced to half of its original value.
Example: 10 mg of a drug is administered. Blood concentration become 5 mg (50%) after 20 minutes. So, plasma half-life = 20 minutes.
Taking the simplest case of a drug which has rapid one compartment distribution and first order elimination, and is given i.v. a semilog plasma concentration-time plot as shown in Fig. below is obtained. The plot has two slopes.
➤Initial rapidly declining (a) phase-due to distribution.
➤ Later less declined (B) phase-due to elimination.
At least two half-lives (distribution t½ and elimination t½) can be calculated from the two slopes. The elimination half-life derived from the ẞ slope is simply called the ‘half-life’ of the drug.
Clinical Importance of Plasma half life
Plasma half-life gives idea about:
1. Duration of action of drug.
2. Amount of drug to be administered.
3. Frequency of administration.
4. Management of drug overdose.
Plasma half-life depends on following factors
1. Routes of drug administration.
2. Amount of drug administered (dose)
- Low dose phenytoin t½: 10-15 h.
- High dose phenytoin: t½: >60 h
3. Age of the patient: in infancy & old age, renal elimination and hepatic metabolism are generally impaired relative to other age:
4. Plasma protein binding of the drug.
5. Drug Biotransformation.
6. Elimination of drugs.
7. Genetic factors:
- Isoniazide: Slow acetylators t½-3h
- Isoniazide: Fast acetylators t½ – 1.5 h

Clinical Conditions Resulting Increased Drug Half-Life
Half–life of a drug can be increased in the following conditions:
➤ With diminished renal plasma flow, for example:
- Cardiogenic shock.
- Heart failure.
- Haemorrhage
➤ With addition of second drug which displaces from its plasma protein binding, hence increases of distribution of the drug.
- Digoxin + Quinine
• Quinine displaces Digoxin from plasma protein and thereby increases Digoxin half–life. Biological effective half life
The time in which the pharmacological effect of the drug and of any active metabolites has declined of one-half.
Biological elimination half life
The biological half-life is the rime in which the biological effect of a drug declines by one half.
Read more: