Today our topic of discussion is ” Acid Base Balance “. Understanding the complex interactions between acids, bases, and buffers that regulate their impact on the body is fundamental to grasping the concept of acid-base balance.
This balance is a critical aspect of homeostasis – the body’s self-regulating process that maintains stability while adjusting to conditions that are optimal for survival. Acid-base balance specifically refers to the mechanisms the body uses to keep its internal pH within a narrow, slightly alkaline range (7.35 to 7.45). Disturbances in this delicate balance can lead to severe health consequences.
Acid Base Balance : Fluid, Electrolyte, and Acid-Base Balance
The Importance of Acid-Base Balance
The body’s metabolic processes constantly produce acid. For instance, carbon dioxide (CO2), a byproduct of cellular respiration, combines with water to form carbonic acid, a weak acid that dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-). The body must eliminate these acids to maintain pH homeostasis. Failure to do so can lead to acidosis or alkalosis, conditions where the body’s pH deviates from its normal range.
Regulatory Mechanisms
The body maintains its acid-base balance through three primary mechanisms: buffer systems, respiratory compensation, and renal compensation.
- Buffer Systems Buffer systems are the first line of defense against pH changes. They consist of a weak acid and its corresponding base, which can neutralize excess acids or bases. The most significant buffer system in the body is the bicarbonate buffer system. Hemoglobin and proteins also act as buffers, as do phosphates.
- Respiratory Compensation The respiratory system responds to changes in pH by adjusting the rate and depth of breathing. An increase in H+ concentration (decreasing pH) is sensed by central chemoreceptors in the brain and peripheral chemoreceptors in the carotid and aortic bodies, which in turn stimulate the respiratory center to increase ventilation. This heightened breathing expels more CO2, thus reducing the concentration of carbonic acid and raising the pH back toward normal. Conversely, a decrease in H+ concentration (increasing pH) will slow down respiration, retaining CO2 and lowering pH.
- Renal Compensation The kidneys maintain acid-base balance by excreting or retaining H+ and bicarbonate ions. They can take hours to days to respond but provide a more powerful and long-lasting effect compared to respiratory compensation. In acidosis, the kidneys excrete more H+ and reabsorb more HCO3-, while in alkalosis, they do the opposite.
Acid-Base Disorders
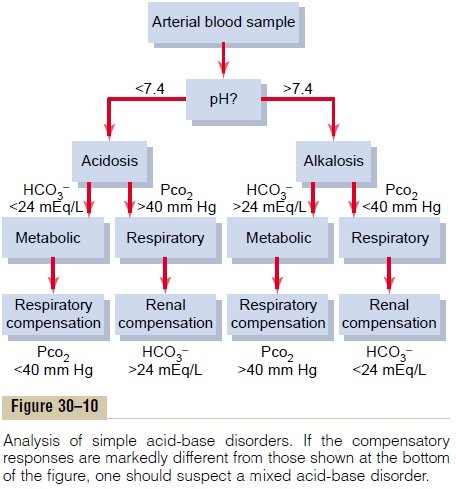
Imbalances in acid-base homeostasis can result in four primary disorders: metabolic acidosis, respiratory acidosis, metabolic alkalosis, and respiratory alkalosis.
- Metabolic Acidosis Metabolic acidosis occurs when the body produces excessive amounts of acid (as in lactic acidosis) or when the kidneys are not removing enough acid from the body. It can also happen due to a loss of bicarbonate (as in diarrhea).
- Respiratory Acidosis Respiratory acidosis develops when there is an accumulation of CO2 due to reduced gas exchange or hypoventilation. Conditions such as chronic obstructive pulmonary disease (COPD) can impair the ability to exhale CO2 efficiently.
- Metabolic Alkalosis Metabolic alkalosis is often the result of excessive loss of H+ (from prolonged vomiting, for instance) or an increase in HCO3- (such as from ingestion of bicarbonates).
- Respiratory Alkalosis Respiratory alkalosis is caused by hyperventilation, which can result from anxiety, pain, or other conditions that stimulate the respiratory centers to increase breathing, leading to excessive expulsion of CO2.

Clinical Assessment of Acid-Base Balance
Healthcare providers typically assess acid-base balance by measuring the arterial blood gas (ABG), which provides a direct indication of respiratory and metabolic function. It includes the measurement of pH, partial pressure of CO2 (PaCO2), and bicarbonate (HCO3-). Anion gap calculation is also essential in cases of metabolic acidosis to differentiate between its causes.
Acid-Base Balance and Electrolytes
Electrolytes like sodium (Na+), potassium (K+), chloride (Cl-), and calcium (Ca2+) play a pivotal role in maintaining acid-base balance. For instance, K+ can exchange with H+ in the renal tubules, affecting acid excretion. Na+ reabsorption is often accompanied by H+ secretion or HCO3- reabsorption, also impacting acid-base status.
The Importance of Acid-Base Balance in Disease States
Acid-base disturbances often occur in the context of broader health issues. For example, diabetic ketoacidosis (DKA) is a type of metabolic acidosis that arises from the accumulation of ketones due to inadequate insulin levels. Sepsis can lead to lactic acidosis due to hypoperfusion and subsequent anaerobic metabolism. Pulmonary diseases, such as emphysema, may lead to respiratory acidosis by impeding CO2 elimination.
Nutrition and Acid-Base Balance
Diet can influence systemic pH. A diet high in animal protein tends to produce more acid, whereas a diet rich in fruits and vegetables may increase the alkalinity due to the generation of bicarbonate during metabolism. Nevertheless, the body’s regulatory systems are typically robust enough to manage the acid-base impact of a balanced diet.
Fluid Balance and Acid-Base Homeostasis
The interplay between fluid and acid-base balance is crucial. Fluid loss, as through diarrhea or vomiting, can lead to an electrolyte imbalance, which may disturb the acid-base balance. Similarly, excessive IV fluid administration can dilute electrolytes and H+ concentration, affecting pH.
Conclusion
Acid-base balance is a complex yet elegantly orchestrated aspect of the body’s homeostatic mechanisms. It involves intricate feedback systems that monitor and adjust the body’s pH level, reflecting the dynamic interplay between respiratory, renal, and buffer systems. Understanding and managing acid-base disorders are critical in clinical medicine and require a systematic approach to diagnosis and treatment.
While the body has its own powerful regulatory systems to maintain this balance, our lifestyle choices, particularly in diet and health management, can influence these mechanisms. Ongoing research continues to uncover the nuances of acid-base homeostasis and its implications for health and disease.
Read more:
