Today our topic of discussion is ” Disorders of Acid Base Balance “. The human body meticulously maintains its internal environment to support essential biochemical processes. One of the most vital aspects of this homeostasis is the maintenance of acid-base balance, which is crucial for normal cellular functions.
Disorders of acid-base balance can significantly impact the fluid and electrolyte status of the body, leading to potentially life-threatening conditions. This article will delve into the nature of these disorders, their pathophysiology, clinical presentation, diagnostic strategies, and management principles.
Disorders of Acid Base Balance : Fluid, Electrolyte, and Acid-Base Balance
Understanding Acid-Base Balance
Before we explore the disorders, it’s critical to understand what acid-base balance is. The body’s fluids contain acids and bases, which are carefully regulated to keep the pH of the body’s environments within a narrow range, typically between 7.35 and 7.45 for blood. This balance is achieved through a complex interplay between the lungs, kidneys, and various buffer systems within the body.
Acids are substances that donate hydrogen ions (H+) when dissolved in water, while bases accept them. The body’s metabolism generates a constant production of acids, mainly carbon dioxide (CO2) and hydrogen ions. These must be neutralized or excreted to maintain pH levels.
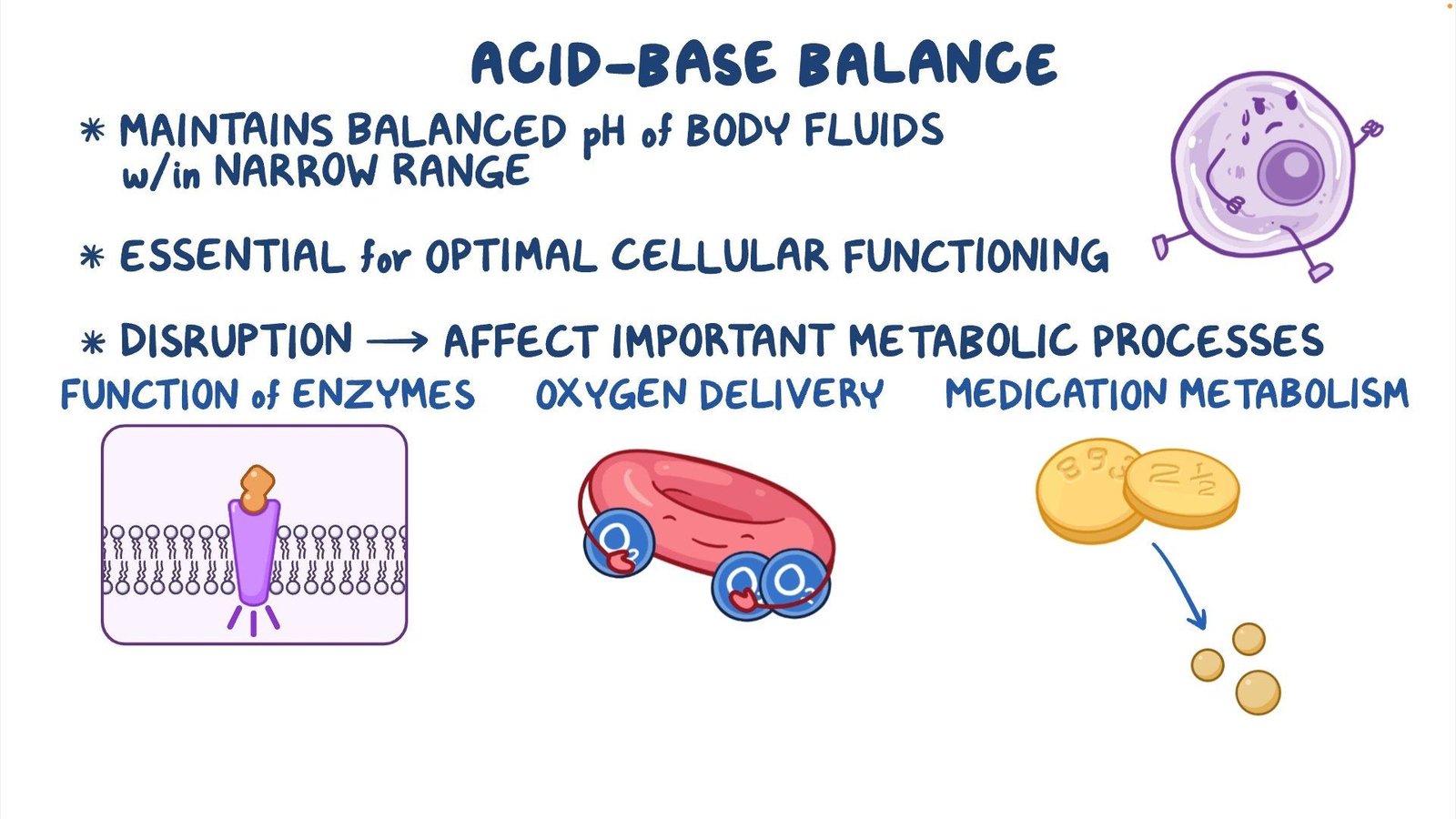
Types of Acid-Base Disorders
There are four primary disorders of acid-base balance:
- Metabolic Acidosis
- Respiratory Acidosis
- Metabolic Alkalosis
- Respiratory Alkalosis
- Metabolic Acidosis
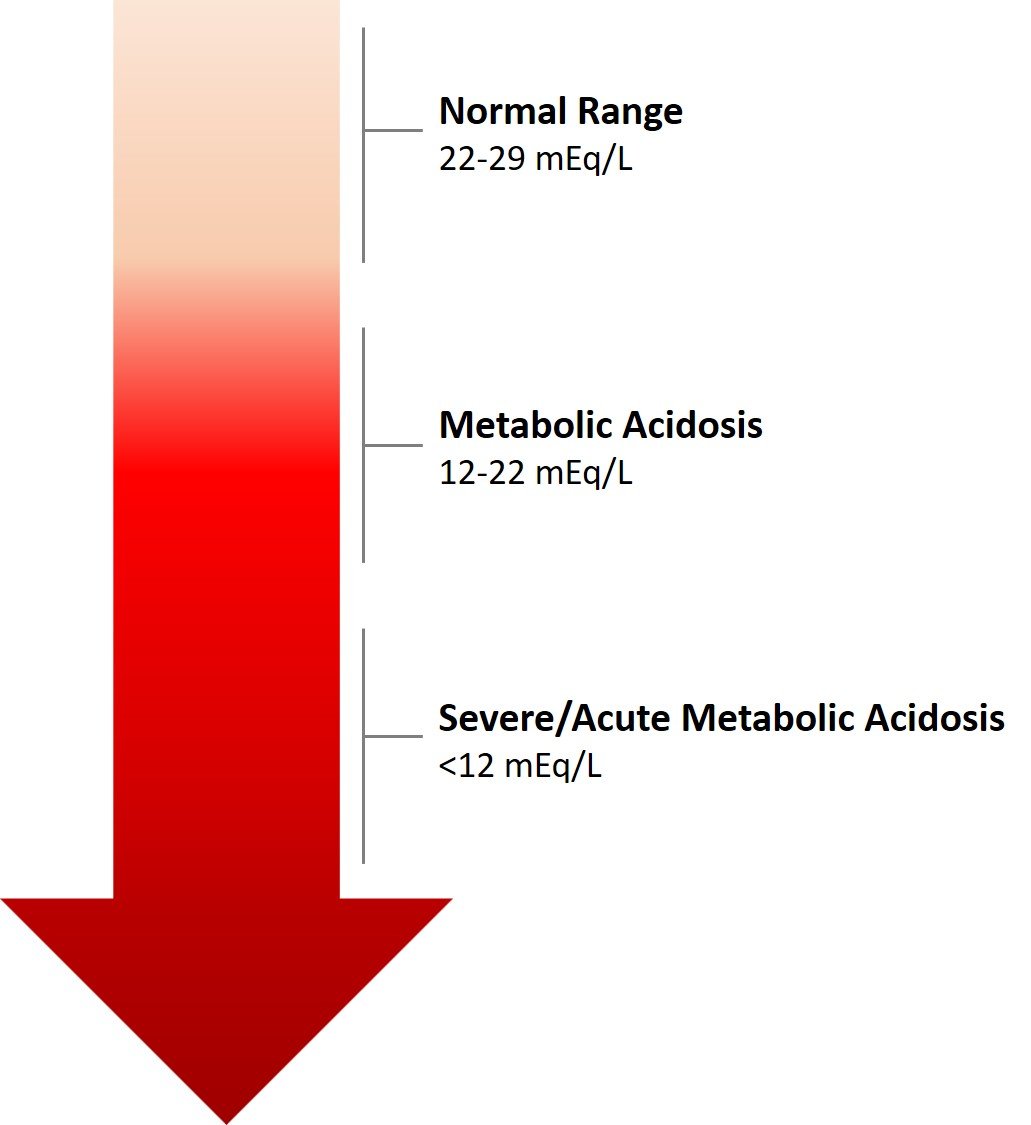
1. Metabolic acidosis
Metabolic acidosis is characterized by a decrease in serum bicarbonate (HCO3-) concentration, an increase in hydrogen ion concentration, and a resultant decrease in blood pH. Causes of metabolic acidosis can be categorized as those that increase acid production (e.g., ketoacidosis, lactic acidosis), those that result in bicarbonate loss (e.g., diarrhea), and those due to reduced renal acid excretion (e.g., chronic kidney disease).
In metabolic acidosis, the body attempts to compensate through respiratory mechanisms. The central chemoreceptors stimulate the respiratory center to increase the rate and depth of breathing (hyperventilation), which lowers the partial pressure of carbon dioxide (PaCO2) and raises the blood pH toward normal.
Clinical presentation may include Kussmaul respiration (deep, labored breathing), lethargy, confusion, and if severe, shock or death. The anion gap, which is the difference between measured cations and anions in serum, is an essential tool for categorizing metabolic acidosis. A high anion gap indicates an accumulation of acids, whereas a normal anion gap suggests a loss of bicarbonate.
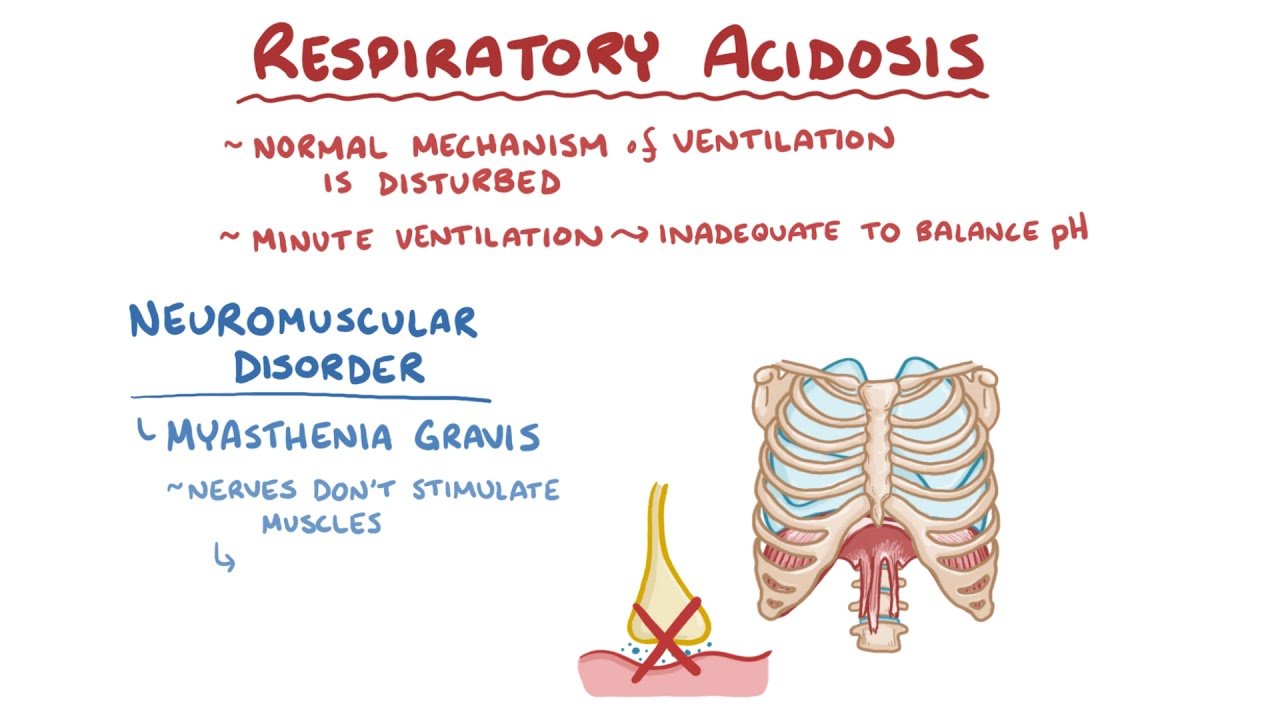
2. Respiratory Acidosis
Respiratory acidosis occurs when there is an accumulation of CO2 due to hypoventilation. This may be due to lung diseases, chest wall abnormalities, respiratory muscle weakness, or central nervous system depression. The increased CO2 reacts with water to form carbonic acid, which dissociates into hydrogen ions and bicarbonate, leading to a reduction in pH.
Kidneys compensate for respiratory acidosis by increasing the reabsorption of bicarbonate and secretion of hydrogen ions, a process that takes several days. Symptoms may include headaches, confusion, lethargy, and in severe cases, signs of raised intracranial pressure due to vasodilation of cerebral vessels.
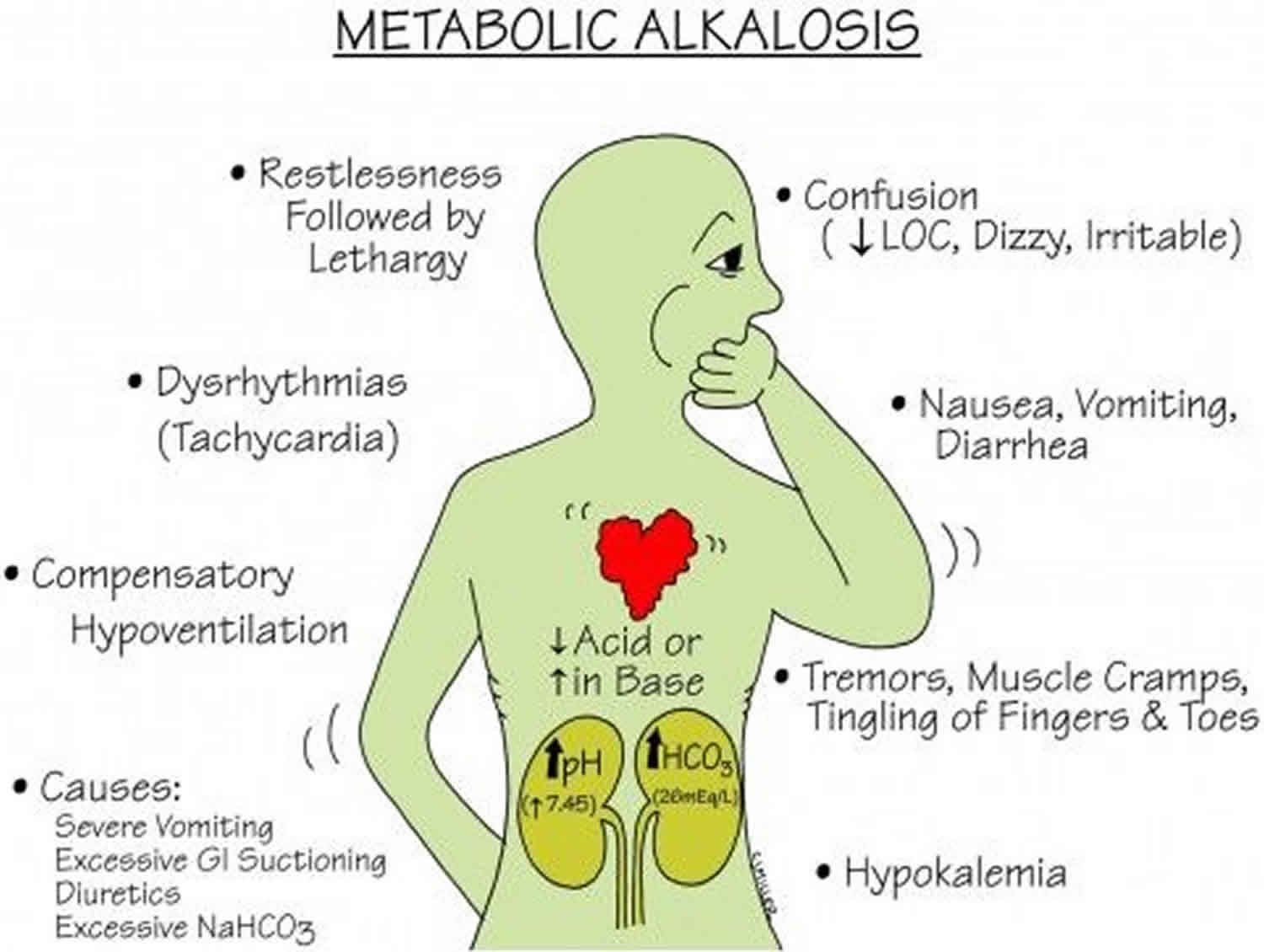
3. Metabolic Alkalosis
Metabolic alkalosis is a condition characterized by an increase in bicarbonate or a decrease in hydrogen ion concentration, leading to an increase in blood pH. It is often caused by excessive loss of gastric acid (from vomiting or nasogastric suction), diuretic therapy, or an excess intake of bicarbonate. The compensatory mechanism includes hypoventilation by the respiratory system to increase CO2 and decrease pH.
Patients with metabolic alkalosis may exhibit symptoms like muscle cramps, twitching, weakness, and in severe cases, tetany due to associated hypocalcemia. They may also have symptoms of concomitant volume depletion, such as hypotension and tachycardia, due to diuretic use or vomiting.
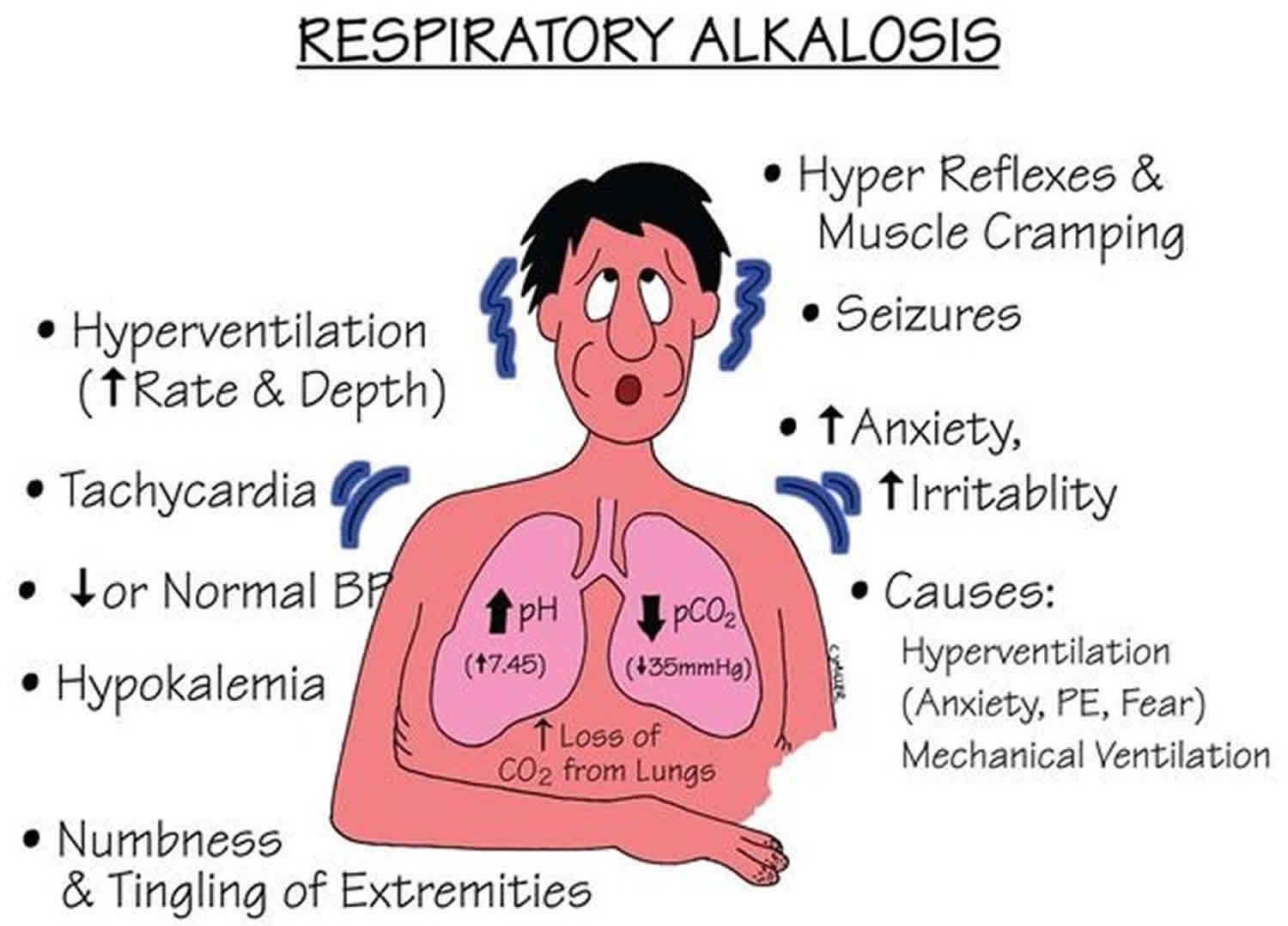
4. Respiratory Alkalosis
Respiratory alkalosis is the result of excessive loss of CO2 through hyperventilation. This may be due to physiological stimuli (e.g., hypoxemia, pain), psychological stimuli (e.g., anxiety), or iatrogenic causes (e.g., mechanical ventilation). The reduction in CO2 leads to a decrease in hydrogen ion concentration and an increase in pH.
The kidneys respond by decreasing bicarbonate reabsorption and hydrogen ion secretion. The clinical presentation of respiratory alkalosis may include lightheadedness, numbness, tingling of the extremities, and in severe cases, seizures.
Diagnostic Strategies
The arterial blood gas (ABG) analysis is the cornerstone of diagnosing acid-base disorders. It provides a direct measurement of the pH, PaCO2, and bicarbonate levels in the blood. In combination with a thorough clinical assessment and additional laboratory tests such as serum electrolytes, lactate, and ketone levels, healthcare professionals can determine the nature and cause of the acid-base disorder.
Management Principles
Treatment of acid-base disorders focuses on addressing the underlying cause and restoring normal acid-base balance. For example, therapy for metabolic acidosis may involve administering bicarbonate in cases of severe acidemia or treating the underlying cause, such as insulin for diabetic ketoacidosis. In respiratory disorders, managing the patient’s ventilation is key, whether by adjusting mechanical ventilation settings or providing medications to improve respiratory drive or lung function.
Electrolyte imbalances often accompany acid-base disorders and must be corrected concurrently. For instance, potassium levels can fluctuate significantly in acid-base disorders and require careful monitoring and management.

Conclusion
Disorders of acid-base balance can be complex and challenging to manage, requiring an integrated understanding of the underlying physiology, pathophysiology, and clinical implications. A systematic approach to diagnosis, including the interpretation of ABG results in the context of the patient’s clinical status, is essential for effective management. Prompt and appropriate treatment is crucial to prevent the significant morbidity and mortality associated with these disorders.
Fluid, electrolyte, and acid-base balance are intricately linked, and disturbances in one can affect the others. Thus, a comprehensive approach that considers all these factors is required for optimal patient care. With advances in monitoring and treatment modalities, healthcare providers can better address these critical issues, improving outcomes for patients with acid-base imbalances.
Read more:
