Blood plasma pH & hydrolysis – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Blood plasma pH & hydrolysis
pH
pH is defined as the negative logarithm of the hydrogen ion concentration to the base 10 in moles per liter (mol/L). The term pH was first introduced in 1909 by Sorensen.
➤ ‘P’ indicates the negative logarithm to base 10.
➤ ^ prime H’ indicates the hydrogen ion concentration.
i.e. pH =-log[H+ ]
=log[ 1/H+ ]
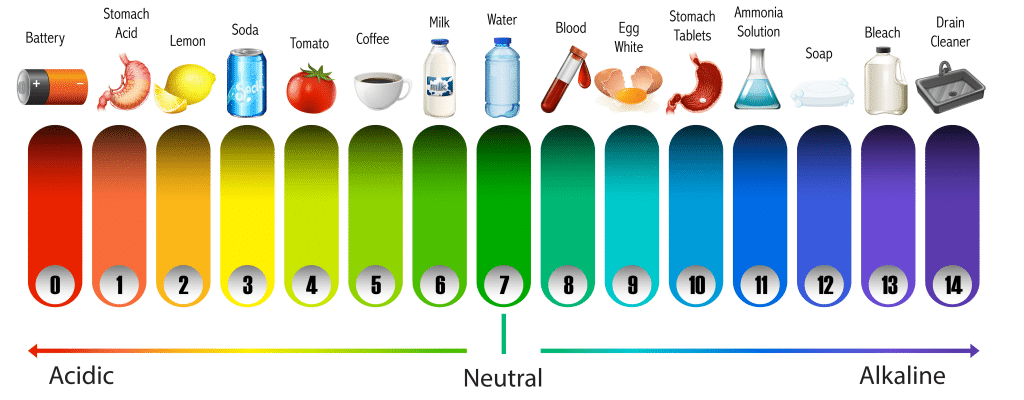
Some criteria about pH: PH Range = 0-14.
➤ When P ^ H is 7: It is Neutral.
➤ When P^ H is > 7 It is Alkaline.
➤ When P ^ H * is < 7 : It is Acidic.
What do you mean by the P ^ H of a solution is less than 7 or is greater than 7?
When the P ^ H of a solution is less than 7, the hydrogen ion concentration of that solution is higher than that of a neutral solution and it becomes acidic. On the contrary, when the P ^ H is greater than 7, the hydrogen ion concentration is lesser than that of a neutral solution and it becomes alkaline.
Name the systems of human body that controls or preserve P ^ H?
The systems that controls PH are as follows –
1. Buffer system.
2. Respiratory system.
3. Renal system
Name the method to measure pH of a solution?
The methods to measure pH are :
a) Non-specific methods.
b) Specific methods
Non-specific methods can be divided into following classes-
- pH Paper.
- Litmus paper.
- Indicators.
- Buffer
Again Specific methods can also be divided into following classes
- pH meter.
- Hydrogen electrodes.
- Gas electrodes.
- Calomel electrodes. Etc.
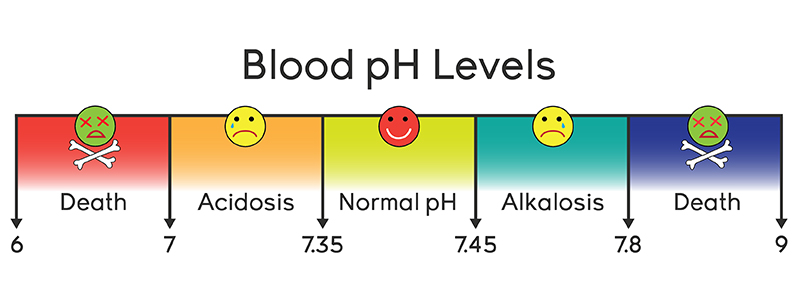
Maintenance of normal pH in a normal person:
Normal pH of the arterial blood is average 7.4, which must be maintained within a limit (7.35 – 7.45) for normal biological activity
Two types of metabolic acids are produced during normal activity-
➤ Volatile acids – e.g. H2CO3
➤ Non-volatile acidse.g. H2SO4, H3PO4, Lactic acid etc.
These acids tend to decrease normal pH. So the following three primary systems act in the body which maintain the normal pH –
- Buffer system: acts within seconds (1st line defense).
- Respiration: acts within minutes (2nd line defense).
- Kidney: acts within hours to days (3rd line defense)
Buffer system :
The base component of buffer combines with free Ht of metabolic acids and is converted into weak acids that dissociates very little. Thus buffers prevent abrupt pH change. But buffers cannot remove the Ht from the body. They only keep the H* ‘tie up’ until lungs & kidney remove the H+ from the body.
B (buffer base) + H+ (from metabolic acid) -> HB (weak acid)
e.g. HCO3 (buffer base) + H+ (from metabolic acid) -> H2CO3 (weak acid)
Respiration:
Lungs excrete H2CO3 (volatile acid) in the form of CO2 through expiration. CO2 is produced by the end product of metabolism that binds with H2O to form H2CO3 in the blood. H2CO3 comes to lungs and dissociates into H₂O & CO2. The CO₂ is then excreted through the lungs.
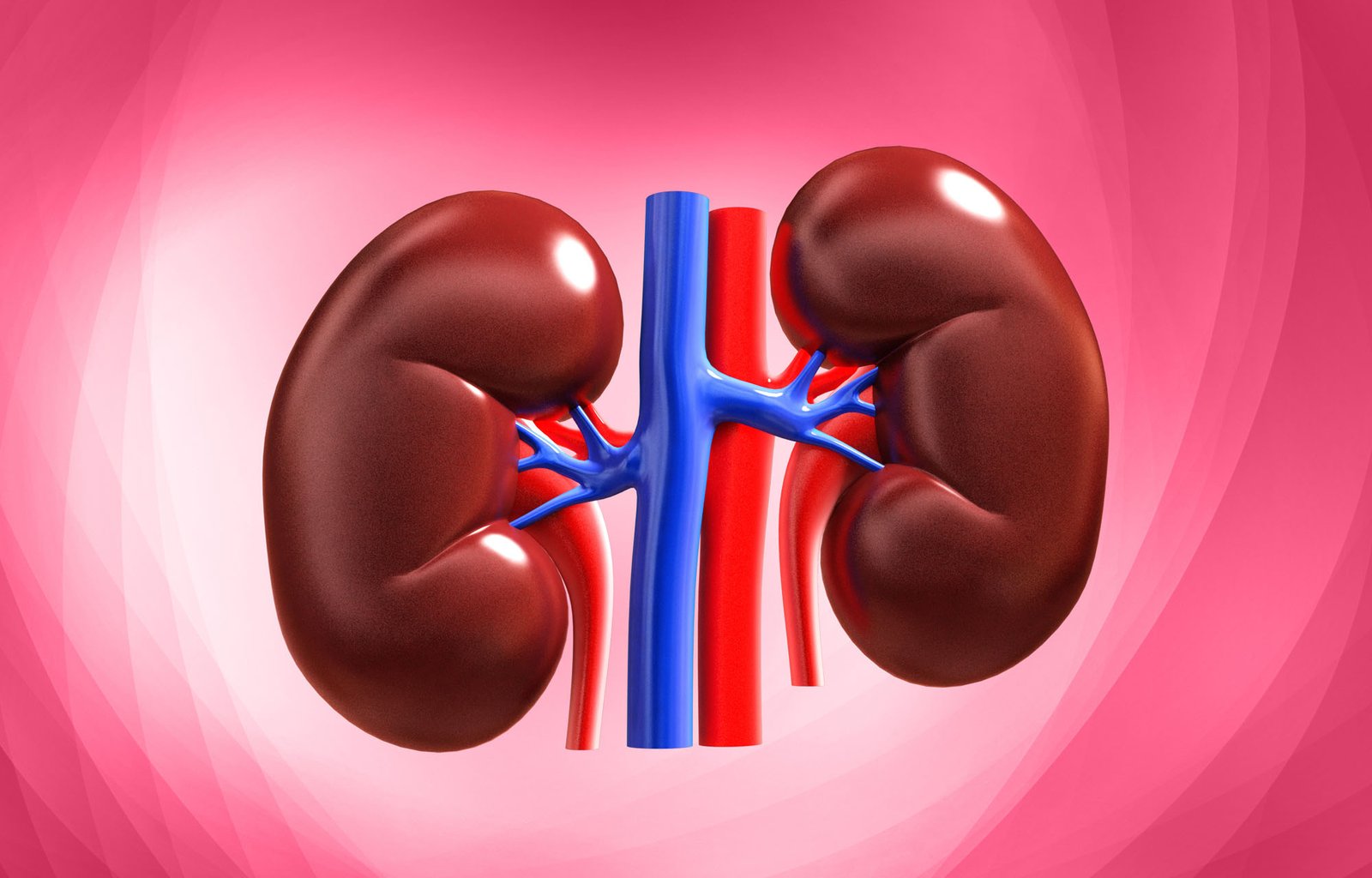
Kidney:
Kidney maintains normal pH by excreting either acidic or alkaline urine. This occurs by –
- Reabsorption of filtered HCO3.
- Generation of new HCO3-.
- Excretion of non-volatile acids
Importance of pH:
- pH is the indicator of a solution whether it is acidic or basic e.g.
✓ pH = 7 indicates neutral solution.
✓ pH below 7 (0-7) indicates acidic and
✓ pH above 7 (7-14) indicates alkaline solution
- pH should be maintained within normal range for proper activity of an enzyme,
- pH is necessary to maintain the normal activity of vital organs in the body.
Measurement of pH:
pH of a solution can be determine by two ways-
➤ For strong electrolytes, the hydrogen ion concentration [H+] is determined first and then the pH can be calculated by the following formula-
- pH = -log[H+]
➤ For weak electrolytes, the pH can be calculated by the ‘Law of Mass Action’
- Ka x HA = [H+] [A-]
- Ka= [H+][A] / [HA]
Here
- HA Undissociated weak acid.
- Ka Dissociation constant.
- H+ = Cation
- A = Anion.
pH of various fluids:
| Fluid | PH |
| Pure water at 250c temp. | 7 |
| Saliva | 6.0-7.0 |
| Blood | 7.35-7.45 (average 7.4). ➤ Arterial blood: 7.40 |
| Gastric HCL | 0.8 |
| Gastric secretion | 1.0-3.5 |
| Pancreatic secretion | 8.0-8.3 |
| Bile | 7.8 |
| Small intestinal secretion | 7.5-8 |
| Urine | 4.5-8.0 |
| Tears | 7.2 |
| Milk | 6.6-6.9 |
PH Scale
The scale which denotes the P ^ H Marking of the P ^ H scale: 0 to 14. The highest PH of a solution could be 14.
➤ P ^ H = 0i indicates almost pure acid solution.
➤ P ^ H = 14 indicates almost pure alkali solution.
➤ P ^ H = 7i indicates neutral solution.
➤ p ^ H above 7 indicates alkaline solution.
➤ P ^ H * below * 7 indicates acidic solution.
Properties:
- The scale is between 0 & 14.
- Hydrogen ion concentration [H+] cannot be zero.
- The more the Hydrogen ion concentration [ H +], the less is the PH and vice versa.
- Variation in PH scale by 1 unit means ten times variation in hydrogen ion concentration
Examples of pH Conditions: