Today our topic of discussion is ” Body Fluids and Fluid Compartments “. The intricate systems governing body fluids and fluid compartments are fundamental to understanding fluid, electrolyte, and acid-base balance. These balances are crucial for maintaining homeostasis, enabling cellular function, and supporting overall health. This article explores the composition and distribution of body fluids, the regulation of fluid compartments, and the mechanisms of maintaining electrolyte and acid-base equilibrium.
Body Fluids and Fluid Compartments : Fluid, Electrolyte, and Acid-Base Balance
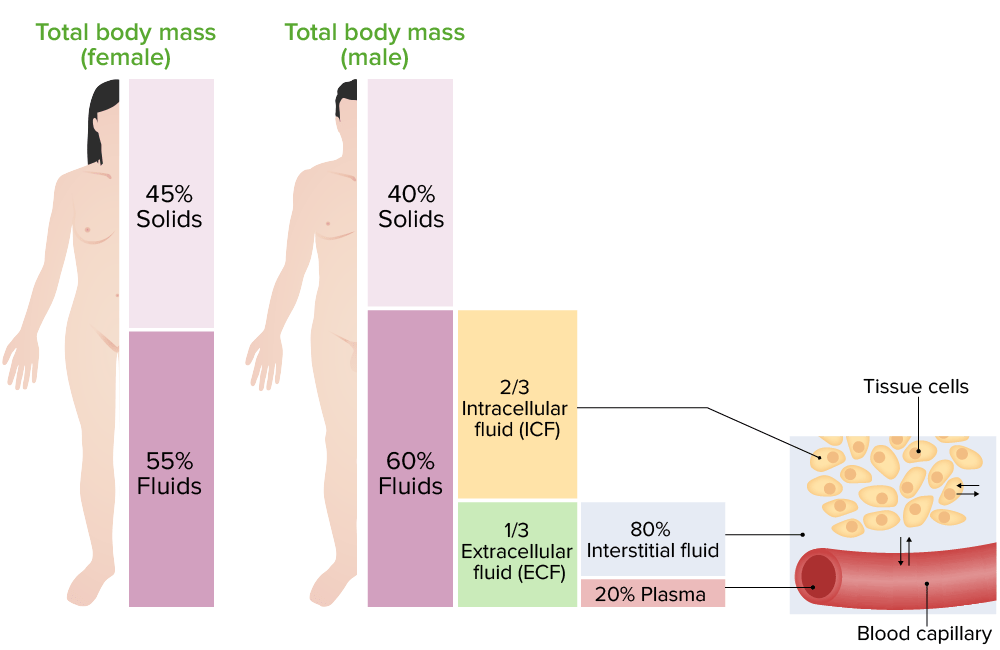
Human life is inextricably linked to the balance of fluids, electrolytes, acids, and bases within the body. Approximately 60% of the adult human body is composed of water, a fundamental component necessary for all forms of life. This water is not uniformly distributed but is compartmentalized into intracellular and extracellular spaces, each playing vital roles in physiological function.
Proper balance within these compartments is essential for various bodily processes, including nutrient delivery, waste removal, and the maintenance of blood pressure. Disruptions in fluid, electrolyte, and acid-base balance can lead to significant clinical conditions.

Body Fluid Composition and Distribution
1.1. Water: The Universal Solvent Water serves as the universal solvent in which electrolytes, nutrients, and waste products are dissolved and transported throughout the body. The distribution of water in the body is dynamic and subject to regulatory processes that ensure homeostasis.
1.2. Fluid Compartments The body’s water is divided into two major compartments: the intracellular fluid (ICF) compartment and the extracellular fluid (ECF) compartment. The ICF contains approximately two-thirds of the body’s water and is found within cells, serving as the primary solvent for intracellular processes. The ECF encompasses all body fluid outside cells, including interstitial fluid, blood plasma, and transcellular fluids. This compartment facilitates the transport of substances to and from cells.
1.3. Electrolytes and Their Roles Electrolytes are minerals in body fluids that carry an electric charge. The primary electrolytes include sodium, potassium, calcium, magnesium, chloride, hydrogen phosphate, and hydrogen carbonate. They are essential for various bodily functions, including nerve impulse conduction, muscle contraction, and the regulation of pH balance.
Regulation of Fluid Compartments
2.1. Selective Permeability of Cellular Membranes Cellular membranes are selectively permeable barriers that regulate the movement of substances between the ICF and ECF. The movement of water and electrolytes across these membranes is controlled by various mechanisms, including diffusion, osmosis, and active transport.
2.2. Osmoregulation Osmoregulation involves the control of water movement between fluid compartments through osmosis, primarily influenced by the concentration of solutes. The body uses osmoreceptors to detect changes in plasma osmolality and adjusts water intake and excretion accordingly.
2.3. Volume and Pressure Regulation The regulation of fluid volume and pressure involves the renin-angiotensin-aldosterone system (RAAS), antidiuretic hormone (ADH), and natriuretic peptides. These hormones and peptides adjust blood volume and pressure through effects on renal function and vascular tone.

Electrolyte Balance
3.1. Sodium Balance Sodium is the major cation in the ECF and is crucial for ECF volume and osmotic equilibrium. Aldosterone regulates sodium reabsorption in the kidneys, while the dietary intake and renal excretion of sodium can influence its balance.
3.2. Potassium Balance Potassium is the primary cation in the ICF and is essential for cell function. The balance of potassium is regulated by aldosterone, which promotes its excretion, and by the distribution between ICF and ECF, which is influenced by factors such as insulin and pH changes.
3.3. Calcium and Phosphate Balance Calcium and phosphate have reciprocal relationships and are regulated by parathyroid hormone (PTH), vitamin D, and calcitonin. These substances control the absorption, excretion, and storage of these electrolytes in bones, kidneys, and intestines.

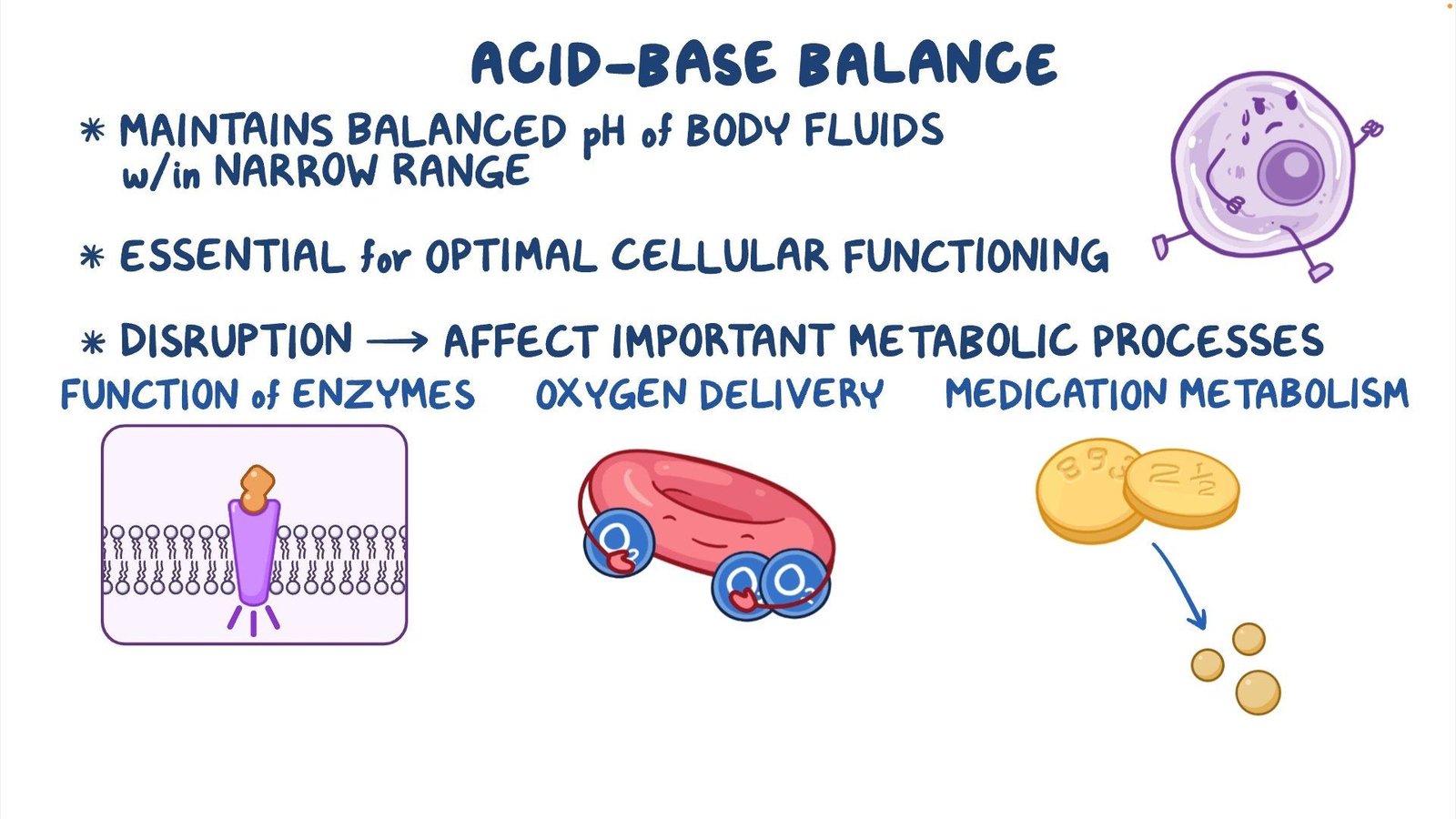
Acid-Base Balance
4.1. pH Regulation The body’s pH is tightly regulated to remain within the narrow range of 7.35 to 7.45. The bicarbonate-carbonic acid buffer system is the primary mechanism for maintaining pH balance within the ECF.
4.2. Respiratory Compensation The respiratory system can adjust the rate and depth of breathing to regulate the exhalation of carbon dioxide, thus altering the carbonic acid concentration in the blood and affecting pH levels.
4.3. Renal Compensation The kidneys contribute to acid-base balance by excreting hydrogen ions and reabsorbing bicarbonate from urine. This renal compensation is slower but more precise and long-lasting than respiratory compensation.
Conclusion
The body’s fluid compartments, electrolyte balance, and acid-base equilibrium are complex and interrelated systems essential for maintaining health. Understanding these systems is crucial for diagnosing and treating disorders that disrupt the balance of body fluids.
It is clear that homeostasis is a finely tuned process requiring the integrated function of multiple organs and regulatory mechanisms. Advances in medical science continue to elucidate these processes, providing deeper insight into how the balance of life is sustained within the milieu of body fluids and compartments.
Read more:
