Classes & Properties of Matter – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Classes & Properties of Matter
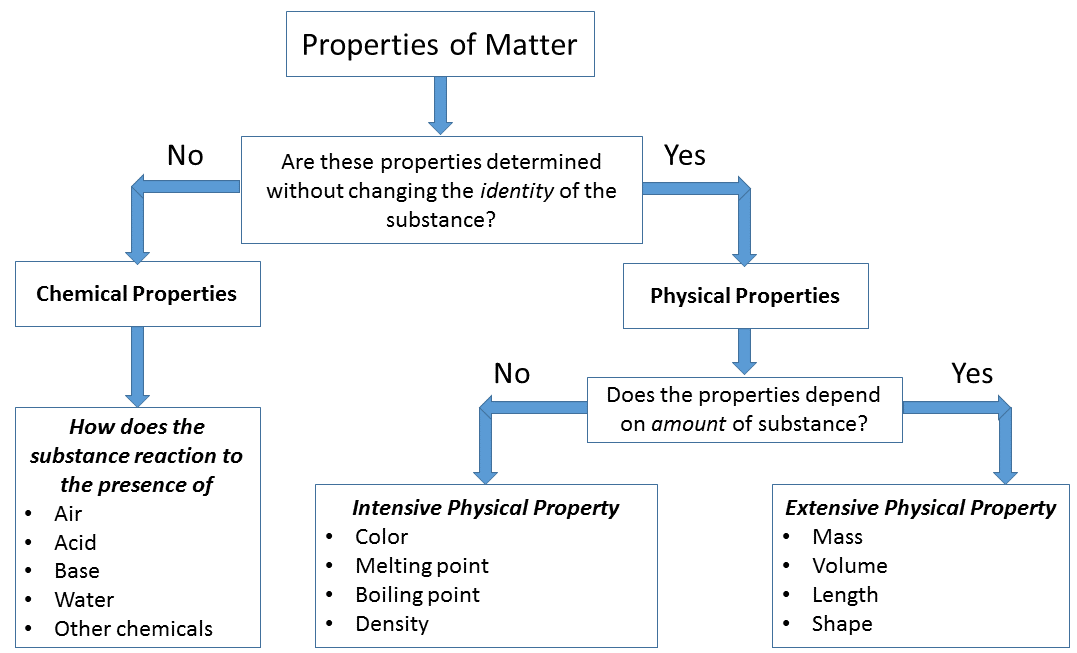
General Properties of Matter:
1. Matter occupies space.
2. Matter has inertia i.e., it is a tendency of bodies in motion to remain in motion, and bodies at rest to remain at rest, unless some external force or friction tends to move it or stop it.
3. Matter has mass (or weight) – mass is the amount of matter or material contained in a body whereas the gravitational pull on a body is called as weight.
4. Matter is indestructible-nothing can be destroyed or created, e.g. if a candle is lighted after sometimes it may disappear but carbon, hydrogen and water vapor produced will be of the same weight as the candle.
Special Properties of Solids:
1. Divisibility – all matter can be broken into smaller pieces by mechanical means.
2. Compressibility-Solids when compressed can be packed up to a smaller volume.
3. Expansion-Solids undergo expansion on heating.
4. Porosity-the power of passing through solids.
5. Elasticity is the property which enables a body which has been compressed to regain its original shape and size after the removal of a deforming force, e.g., the springs, and mattress are elastic.
6. Tenacity-This is measured by the weight required to breath bodies when in the shape of wire.
7. Hardness-The property of solids of resistance to scratching.
Special properties of liquids
Some of the important properties of liquid are given below:
1. A liquid has a definite volume.
- Reason: Intermolecular force of attraction is just strong enough to confine the molecules in a definite space.
2. A liquid has no definite shape and acquires the shape of the container. It can flow from a higher lever to a lower level.
- Reason: Intermolecular force of attraction is weaker in a liquid than in a solid. Liquid molecules can move, slip and slide over each other because their molecular separation is larger. The liquid acquires the shape of the container
3. A liquid is compressible.
- Reason: Distance between the neighboring molecules is larger in a liquid than in a solid
4. A liquid can diffuse into another liquid, but this is much slower as compared to the diffusion of gases.
- Reason: Molecules move faster in a liquid than in a solid but slower as compared to the molecules of a gas.
5. A liquid on heating changes into its gaseous state.
- Reason: Heating increases the intermolecular separation of the liquid molecules but decreases their intermolecular force of attraction. On cooling, vapours lose heat and are converted into liquid
Properties of Surface tension.
1. The greater the surface tension of a liquid the smaller is the area or volume into which it will contract.
2. Surface tension decreases with a rise in temperature.
Applications of surface tension:
1. A liquid with law surface tension i.e., hot water spreads easily and can penetrate microorganisms more readily.
2. Bile lowers the surface tension of water. This is used in the Hay test for determining the presence of bile in urine.
3. When soap and other detergents are dissolved in water, the surface tension is lower than that of water. The solution wets the materials placed in it more readily than would water alone.
The applications of capillary.
1. The rise of spirit in the wick of a spirit lamp.
2. Blotting of ink.
3. The rise of pus and other body fluids in a surgical drain.
4. Water in the fibers of a cloth towel.