Concept about Chemical Bonds – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Concept about Chemical Bonds
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds.
The strength of chemical bonds varies considerably; there are “strong bonds” or “primary bonds” such as covalent, ionic and metallic bonds, and “weak bonds” or “secondary bonds” such as dipole-dipole interactions, the London dispersion force and hydrogen bonding
Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other.
An electron positioned between two nuclei will be attracted to both of them and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei in a bond relatively far apart, as compared with the size of the nuclei themselves.
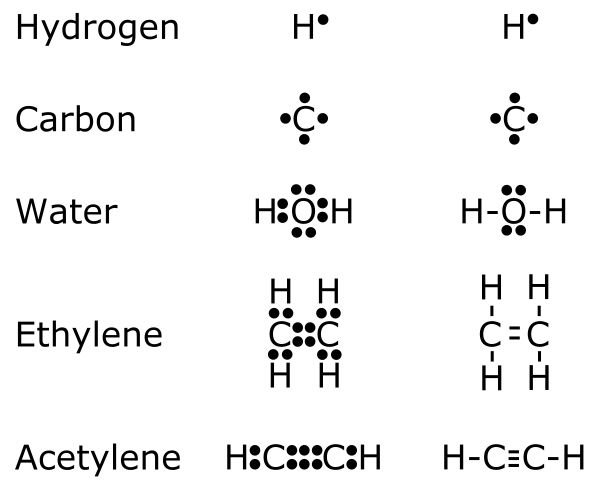
Examples of Lewis dot-style representations of chemical bonds between carbon (C), hydrogen (H), and oxygen (0)
In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases- indeed most of the physical environment around us are held together by chemical bonds, which dictate the structure and the bulk properties of matter.
Definition of Chemical Bond:
A chemical bond is the physical phenomenon of chemical substances being held together by attraction of atoms to each other through sharing, as well as exchanging, of electrons or
electrostatic forces.
or
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.
or
Chemical bonds are the connections between atoms in a molecule. These bonds include both strong intra-molecular interactions, such as covalent and ionic bonds.
Types of Chemical Bonds:
There are two main types of chemical bonds are
1. Covalent bond: Bond in which one or more pairs of electrons are shared by two atoms.
2. Ionic bond: Bond in which one or more electrons from one atom are removed and attached to another atom, resulting in positive and negative ions which attract each other.
Ionic bonds (electrostatic forces that hold ions together…)
Example: Na+Cl-, K+Br
Covalent bonds (result from sharing electrons between atoms…)
Example: H2, NH3
Metallic bonds (refers to metal nuclei floating in a sea of electrons…)
Example: copper, gold
Figure: Example of different types of chemical bonds
Other types of bonds include:
1. Metallic bonds: The properties of metals suggest that their atoms possess strong bonds, yet the ease of conduction of heat and electricity suggest that electrons can move freely in all directions in a metal. The general observations give rise to a picture of “positive ions in a sea of electrons” to describe metallic bonding
2. Hydrogen bonding: In a polar covalent bond containing hydrogen (e.g., an O-H bond in a water molecule), the hydrogen will have a slight positive charge because the bond electrons are pulled more strongly toward the other element. Because of this slight positive charge, the hydrogen will be attracted to any neighboring negative charges. This interaction is called a hydrogen bond.

