Concept about Chemical Reaction – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Concept about Chemical Reaction
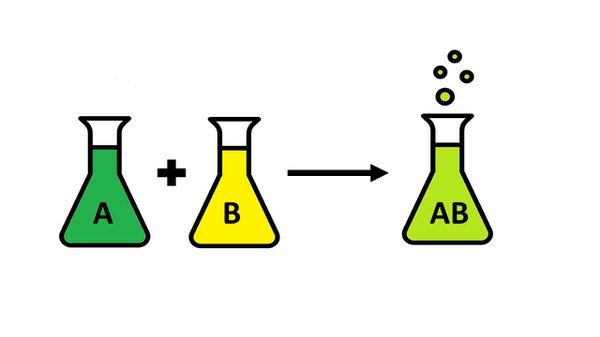
A chemical reaction is typically represented by a chemical equation, which represents the change from reactants to products. The left hand side of the equation represents the reactants, while the right hand side represents the products. A typical chemical reaction is written with stoichiometric coefficients, which show the relative amounts of products and reactants involved in the reaction.
Each compound is followed by a parenthetical note of the compound’s state of 2: (1) for liquid, (s) for solid, (g) for gas. The symbol (aq) is also commonly used in order to represent an aqueous solution, in which compounds are dissolved in water. A reaction might take the following form:
- A(aq)+B(g) C(s)+D(1)(1.1)(1.1)A(aq)+B(g)→C(s)+D(1)
In the above example, AA and BB, known as the reactants, reacted to form CC and DD, the products.
To write an accurate chemical equation, two things must occur:
- Each product and reactant must be written using its chemical formula, e.g., H₂
- The number of atoms of each element must be equal on both sides of the equation. Coefficients are used in front of the chemical formulas in order to help balance the number of atoms, e.g.,
✓ 2Mg+O2→2MgO
Definition of Chemical Reactions:
Chemical reaction is a process in which one or more substances, the reactants, are converted to one or more different substances, the products.
Substances are either chemical elements or compounds.
or
Chemical reactions are the processes by which chemicals interact to form new chemicals with different compositions. Simply stated, a chemical reaction is the process where reactants are transformed into products.