Concept about Gas – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Concept about Gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). A gas mixture would contain a variety of pure gases much like the air.
What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colorless gas invisible to the human observer. The interaction of gas particles in the presence of electric and gravitational fields are considered negligible as indicated by the constant velocity vectors in the image.
Examples of Gases
Whether or not a substance is a gas depends on its temperature and pressure. Examples of gases at standard temperature and pressure include:
- Air (a mixture of gases)
- Chlorine at room temperature and pressure
- Ozone
- Oxygen
- Hydrogen
- Water vapor or steam
List of the Elemental Gases
There are 11 elemental gases (12 if you count ozone). Five are homonuclear molecules, while six are monatomic:
- H_{2} hydrogen
- N_{2} -nitrogen
- O_{2} – oxygen (plus O_{3} is ozone)
- F_{2} fluorine
- C*l_{2} – chlorine
- He – helium
- Ne-neon
- Ar – argon
- Kr-krypton
- Xe – xenon
- Rn – radon
Except for hydrogen, which is at the top left side of the periodic table, elemental gases are on the right side of the table.
Definition of Gas:
A gas is defined as a state of matter consisting of particles that have neither a defined volume nor defined shape.
Or
A gas is a sample of matter that conforms to the shape of a container in which it is held and acquires a uniform density inside the container, even in the presence of gravity and regardless of the amount of substance in the container.
Properties of Gases
1. Particles in a gas are widely separated from each other.
2. At low temperature and ordinary pressure, they resemble an “ideal gas” in which the interaction between the particles is negligible and collisions between them are completely elastic.
3. At higher pressures, intermolecular bonds between gas particles have a greater effect on the properties. Because of the space between atoms or molecules, most gases are transparent. A few are faintly colored, such as chlorine and fluorine.
4. Gases tend not to react as much as other states of matter to electric and gravitational fields. Compared with liquids and solids, gases have low viscosity and low density.
Gas Law:
Pressure in a gas is determined by the number of molecules in a container and their speed. The number of molecules will depend upon the mass and the volume of the molecules, the speed will depend upon the temperature. Quantitatively, the relationship of these factors is given by
PV =mRT
- Where P is the absolute pressure,
- V is the volume
- m is the mass of the gas,
- T is the absolute temperature, and
- R is a constant
This equation represents the general gas law.
The gas equation is the combination of Boyle’s law, laws of Charle’s and Gay-Lussae.
A. Boyle’s law: It states that when the temperature of a gas is kept constant, the volume varies inversely to the pressure exerted upon it, i.e., Pa¹/v; or PV = constant.
B. Charle’s law- It states that when the pressure of a gas is kept constant, the volume is proportional to the absolute temperature, i.e., V a T; or V = RT, where R is constant.
C. Gay-Lussac Law: when the volume of a gas is kept constant, the pressure is proportional to the absolute temperature, i.e., Pa T; or P = KT, where K is constant.
Some Clinical Apparatus Based Upon Gas Laws:-
1. The Respirator: It is an apparatus used to increase and decrease mechanically the pressure in the thorax. It is used chiefly to aid patients suffering from poliomyelitis or other neurologic conditions affecting the muscles of respiration.
2. The Vasculator: It is an apparatus that helps to increase circulation of the peripheral blood vessels.
3. Bronchospirometer: It is a double spirometer that records oxygen consumption of each lung simultaneously and separately. It provides helpful information to the surgeon planning lung surgery since it gives information on both diseased and normal lungs simultaneously.
4. Autoclave: This apparatus is used to sterilize most materials and instruments in the hospital and microbiology laboratory. It works on the Charles and Gay-lussac law, i.e., when volume of a gas remains constant, the pressure is proportional to the temperature. It destroys the microorganisms.
Transport of O₂ from the Lungs to the Tissue Cells:
O2 is transported downhill from lungs to the tissues in 4 steps-
1. Diffusion of O₂ from the alveolar air to the pulmonary blood:
- PO₂ in the alveolus = 104 mm Hg and in the pulmonary capillary at arterial end = A 40 mm Hg
- So, pressure difference = (104-40) mm Hg = 64 mm Hg
- This 64 mm Hg pressure difference causes diffusion of O2 from the alveolus to the pulmonary blood.
2. Transport of O₂ in the blood: O2 is transported in the blood in 2 forms –
- In the form of O₂-Hb. (oxyhemoglobin) : 97%
- In the dissolved state (in plasma) : 3%
3. Diffusion of O₂ from the blood to the interstitial fluid:
- PO2 in the tissue capillaries at arterial end = 95 mm Hg & in the interstitial fluid = 40 mm Hg.
- So, pressure difference = (95-40) mm Hg = 55 mm Hg.
- This 55 mm Hg pressure difference causes diffusion of O2 from the tissue capillaries to the interstitial fluid.
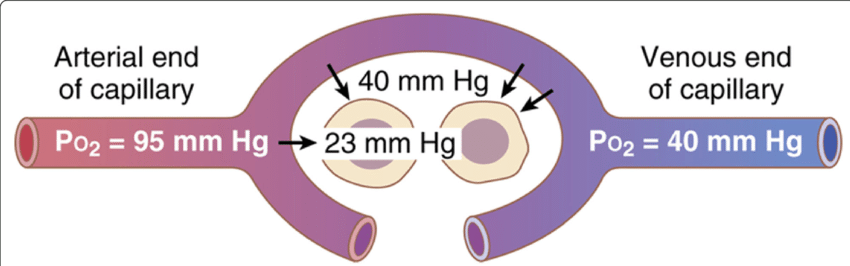
4. Diffusion of O₂ from interstitial fluid to the cells:
- PO2 in the interstitial fluid = 40 mm Hg and in the cells = 23 mm Hg (average).
- So, pressure difference = (4023) mm Hg = 17 mm Hg.
- This 17 mm Hg pressure difference causes diffusion of O2 from the interstitial fluid to the cell
In this way, O₂ is transported from the lungs to the tissue cells.
Transport of CO2:
1. Diffusion of CO2 from the peripheral tissue cells to the interstitial fluid:
- PCO₂ of tissue cells = 46 mm Hg.
- PCO2 of interstitial fluid = 45 mm Hg.
- So, pressure difference = (4645) mm Hg = 1 mm Hg.
- This 1 mm Hg pressure difference causes diffusion of CO2 from the tissue cells to the interstitial fluid.
2. Diffusion of CO2 from the interstitial fluid into the blood:
- PCO2 in interstitial fluid = 45 mm Hg.
- PCO2 in the arterial end of the capillary = 40 mm Hg. So, pressure difference = (4540)mm Hg. = 5 mm Hg.
- This 5 mm Hg pressure difference causes diffusion of CO2 from the interstitial fluid into the blood.
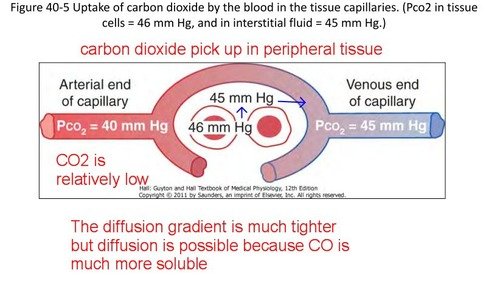
Fig: Exchange of CO₂ between capillary blood & tissue.
3. Transport of CO₂ in the blood: CO₂ is transported in the blood in the following forms –
- In the dissolved state: about 7% CO2 is transported by this way.
- In the form of HCO3: It is the most important method of CO2 transport. About 70% CO2 is transported in this form.
CO2 + H2O <—-> carbonic anhydrase <—-> H2CO3 <—-> H + HCO3 - In the form of carbamino-compound: About 23% CO2 is transported in this form.
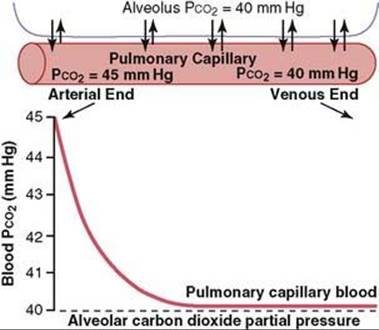
4. Transport of CO₂ from blood to lungs:
- PCO₂ in the arterial end of pulmonary capillary = 45 mm Hg.
- PCO2 of the alveolar air = 40 mm Hg.
- So, pressure difference = (45-40) mm Hg = 5 mm Hg.
- This 5 mm Hg pressure difference causes diffusion of CO2 from the blood into the alveoli.