Concept about Hydrocarbons – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Concept about Hydrocarbons
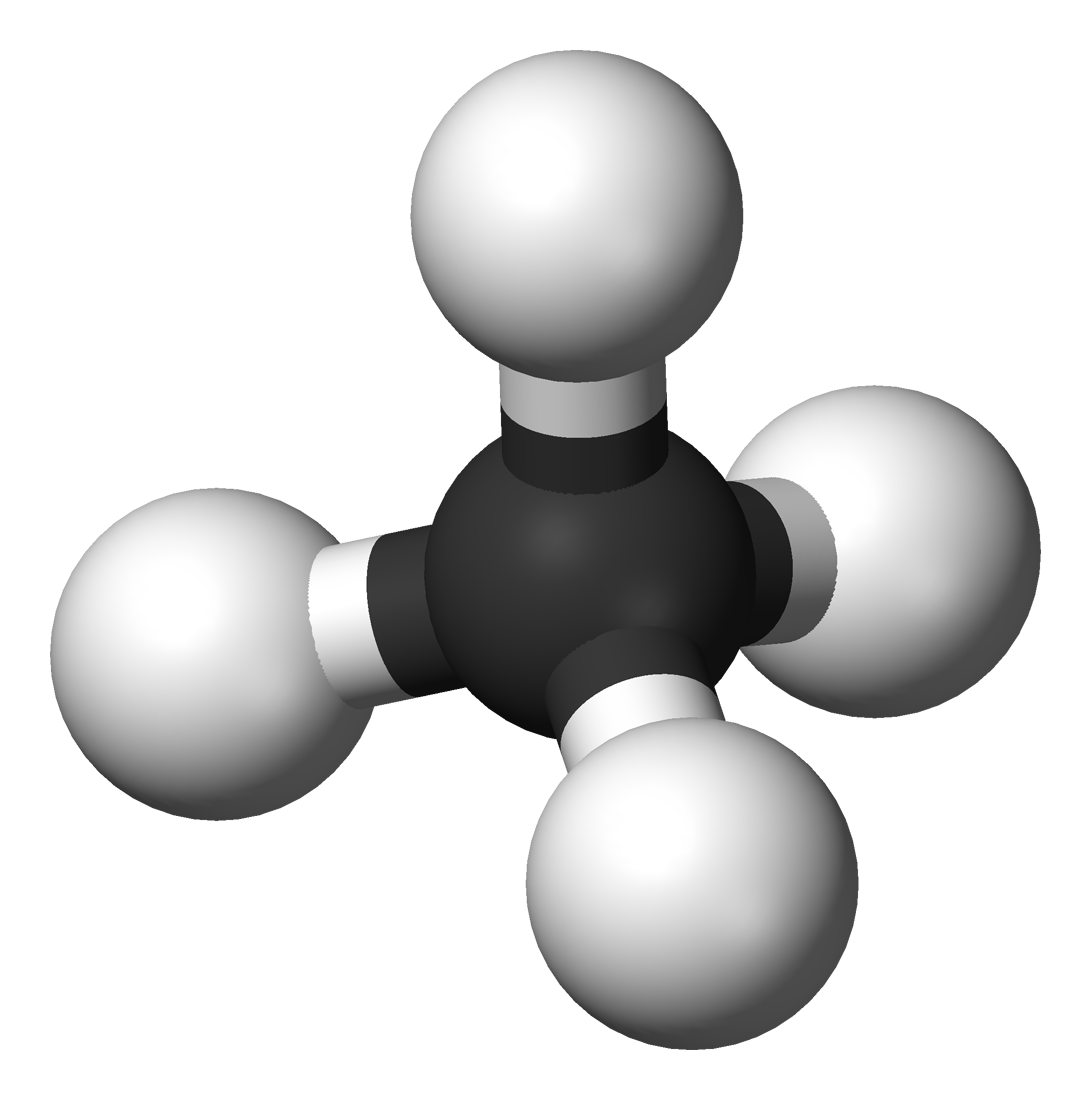
Hydrocarbons are the simplest organic compounds. Containing only carbon and hydrogen,
Or
The chemical compounds which are made by carbon and hydrogen are called hydrocarbons. For eg: Methane, Ethane etc.
Types of Hydrocarbon:
There are two types of hydro carbons. They are:
1. Saturated Hydrocarbons
2. Unsaturated Hydrocarbons
Saturated Hydrocarbons: The hydrocarbons in which a carbon atoms are combined with single bonds is called saturated hydro carbons. For eg: Propane, Pentane etc.
Unsaturated Hydrocarbon: The hydrocarbons in which the carbon atoms are bounded together by a triple or double covalent bond are called unsaturated hydro carbons. For Eg: Ethylene, Acetylene etc
| Saturated Hydrocarbon | Unsaturated Hydrocarbon |
| In them the carbon atoms are bounded together by a single covalent bond. | In them the carbon atoms are bounded together by a triple or double covalent bond. |
| They have only one group which is alkane | They have two groups which is alkene and alkyne |
| They are less active | They are more active |
| Eg: Methane, propane | Eg: Ethene, Propene |
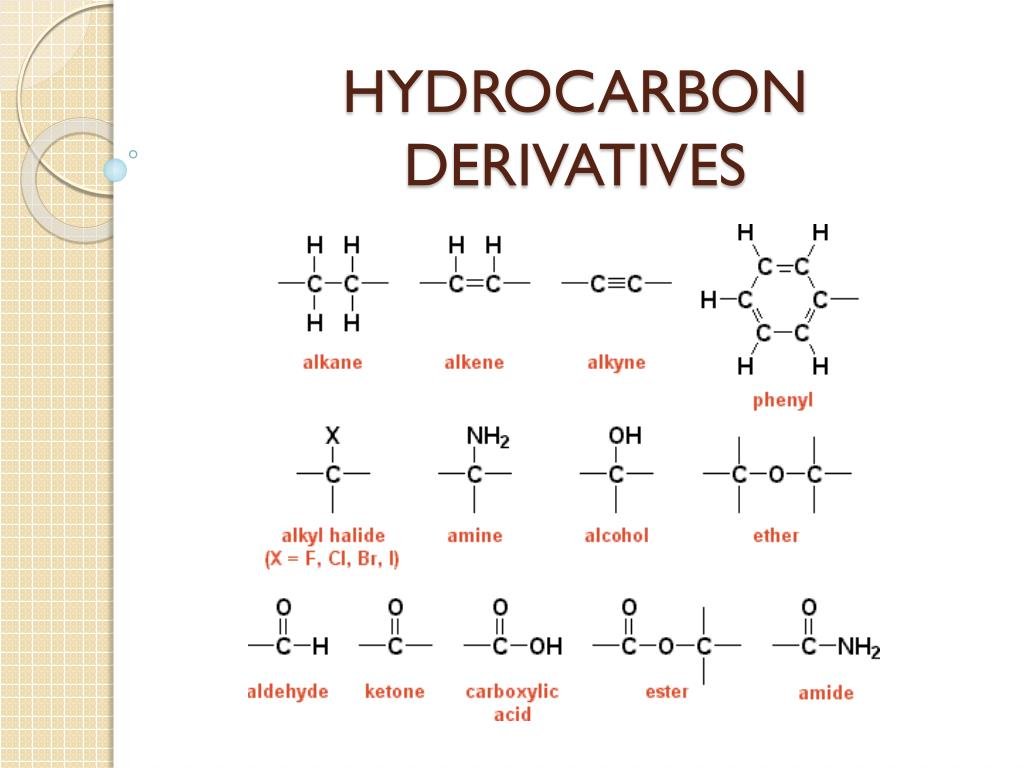
Name of Hydrocarbon Derivatives: