Concept about Radioactivity – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Concept about Radioactivity
Radioactivity refers to the particles which are emitted from nuclei as a result of nuclear instability. Because the nucleus experiences the intense conflict between the two strongest forces in nature, it should not be surprising that there are many nuclear isotopes which are unstable and emit some kind of radiation. The most common types of radiation are called alpha, beta, and gamma radiation, but there are several other varieties of radioactive decay
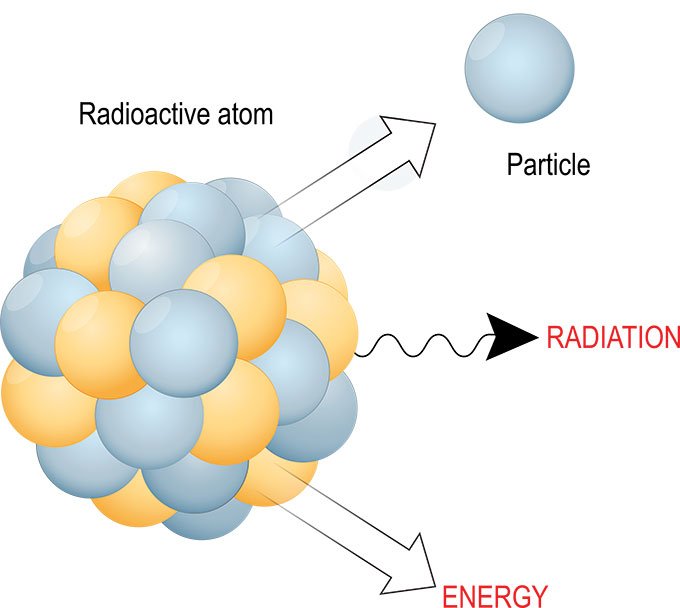
A radioactive atom is one that spontaneously emits energetic particles or waves (known as radiation). This radiation is emitted when an unstable (i.e. radioactive) nucleus transforms to some other nucleus or energy level. Imagine a big ball made of magnets that’s spinning really fast. Sometimes a few pieces of the magnet will shoot out and hit the wall. That’s kind of what radiation is like.
Definition of Radioactivity/Radioactive Decay:
Radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction.
or
Radioactive decay (also known as nuclear decay, radioactivity or nuclear radiation) is the process by which an unstable atomic nucleus loses energy by emitting radiation, such as an alpha particle, beta particle with neutrino or only a neutrino in the case of electron capture, or a gamma ray or electron in the case of internal conversion.
Types of Radioactivity:
There are mainly three types of particles or waves that may shoot out of a radioactive nucleus.
1. Alpha particles (a),
2. Beta particles, (β), and
3. Gamma rays (y)
Alpha Particles
Named alpha because they were the first to be discovered, these particles are made up of 2 protons and 2 neutrons: the helium nucleus. Often, large atoms decay by emitting an energetic alpha particle. These particles are relatively large and positively charged, and therefore do not penetrate through matter very well. A thin piece of paper can stop almost any alpha particle. However, the particles cause extreme damage of materials that they stop in by displacing atoms as they slow. Paper under sustained alpha-irradiation would degrade.
Beta Particles
Beta particles are energetic electrons that are emitted from the nucleus. They are born when a neutron decays to a proton. Since neutrons are neutral particles and protons are positive, conservation of charge requires a negatively charged electron to be emitted. Some isotopes decay by converting a proton to a neutron, thus emitting a positron (an anti-electron). These particles can penetrate matter more than can alpha particles, and it takes a small aluminum plate to stop most beta particles.
Gamma rays
Gamma rays are photons that are emitted from the nucleus. Often an atom in an excited state will de-excite by emitting a gamma ray. Gamma rays are similar to light waves and x-rays, except they are usually much higher frequency and consequently, more energetic. This radiation has no charge, and can penetrate most matter easily, requiring lead bricks for shielding.
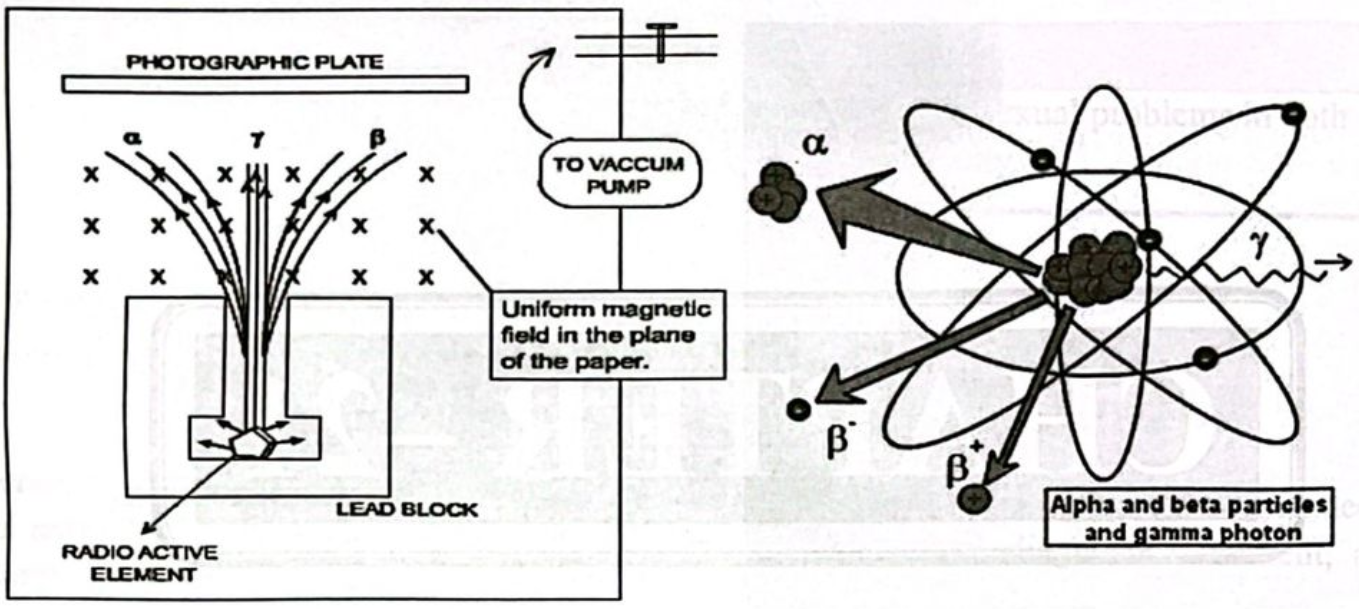
Properties of Alpha Particles (a):
- a particles are positively charged helium nucleus
- Less penetrating power.
- Affected by electric and magnetic fields,
- Effect on photographic plate is feeble,
- Ionize the gas through which they pass.
Properties of Beta Particles (B):
- Fast moving negatively charged electrons. Penetrating power greater than a particles (rays).
- Strongly deflected by electric and magnetic fields.
- Effect on photographic plate is appreciable.
- Strong ionization power.
- Travel in vacuum with the speed of light.
Properties of Gamma Rays (y).
- Have no charge.
- Electromagnetic waves of short wave lengths;
- Not reflected by electric and magnetic fields,
- Have no effect on photographic plate,
- Strong penetrating power.
- Travel in vacuum with the speed of light.