Electrolytes and Body Fluids – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
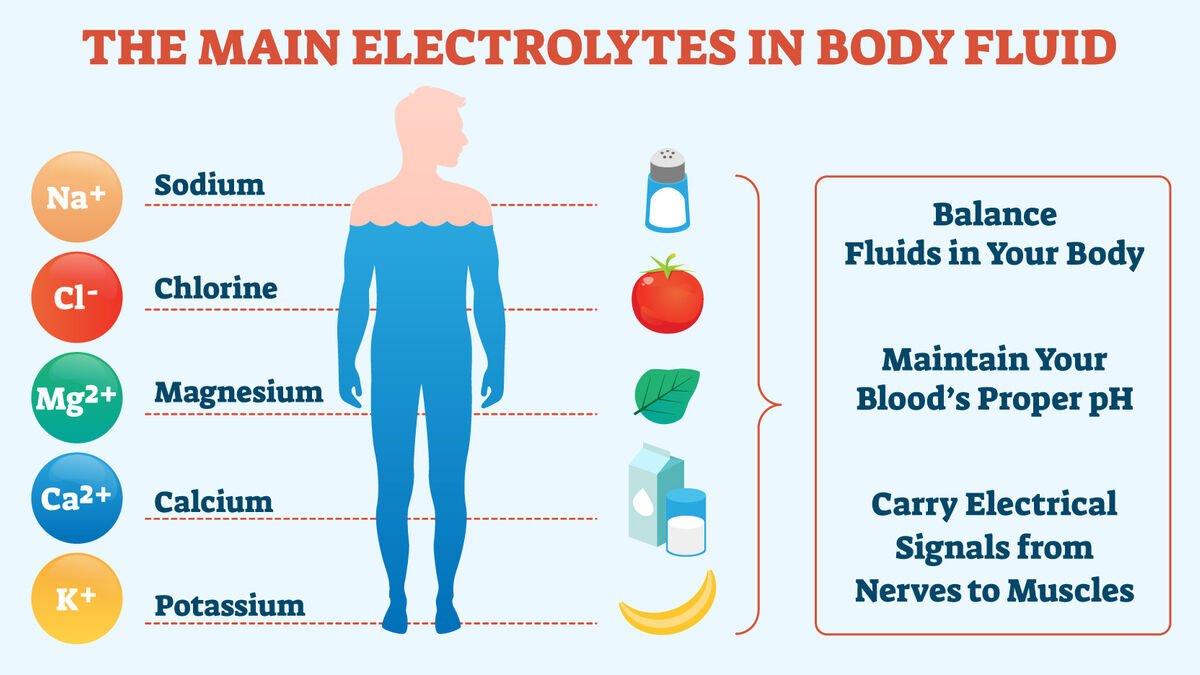
Electrolytes and Body Fluids
The major electrolytes in the plasma of clinical importance are-
➤ Na+ : 135-146 mmol/L.
➤ K+: 95 106 mmol/L.
➤ Cl: 95-106 mmol/L.
➤ HCO3-: 22-30 mmol/L.
➤ Ca++: 2.4 mmol/L.
➤ H: 40 mmol/L.
Note:
Significance of electrolytes:
➤ Na+: It is the major determinant of plasma osmolarity
➤ K+: It is the major determinant of intracellular osmolarity
➤ HCO3: It reflects the acute changes in the acid-base balance
➤ Ca++: It is responsible for many important physiological processes e.g. coagulation cascade, cardiac contraction, neurotransmitter release etc.
Major electrolyte disturbances in the body:
1. Hyponatremia.
2. Hypernatremia.
3. Hypokalemia.
4. Hyperkalemia.
5. Hypocalcemia.
6. Hypercalcemia.
What are the physiological solutions?
Isotonic solution is called physiological solution,
Physiological solutions are:
➤ Solution of 0.9% NaCl.
➤ Solution of 5% glucose.
➤ Solution of 3.8% sodium citrate
Uses of physiological solution (0.9% NaCl):
➤ Correction of dehydration.
➤ Treatment of the burning & hemorrhage.
➤ Used in dressing.
Maintenance of ECF Na+ concentration:
Normal ECF Na conc. is 142 mEq/L (average). ECF Na conc. is maintained by the following mechanisms:
Na+ balance:
➤ Daily Na+ intake: 100-200 mmol.
➤ Daily Na+ output: 100-200 mmol.
➤ Route of Nat excretion/output:
- Urine: 90%.
- Sweat: 5%.
- Stool: 5%.
Na+ balance in ECF is controlled by the following factors –
- Osmoreceptor – ADH mechanism.
- Thirst mechanism.
- Aldosterone concentration.
- Angiotensin II.
- Atrial Natriuretic Factor (ANF).
- Salt – Appetite mechanism.
- Tubular load of Nat.
- Sympathetic stimulation.
Osmoreceptor-ADH mechanism:
↑ Na+ concentration in ECF
↓
↑ ECF osmolarity.
osmoreceptors in supraoptic nuclei of hypothalamus
↓
↑ ADH secretion from posterior pituitary.
↓
↑ Plasma ADH
↑ Permeability of water in DCT, CT & CD in kidney
↓
↑ Water reabsorption.
↓
↑ ECF volume.
↓
↓Nat conc. in ECF.
Again,
Na+ concentration in ECF
↓
↓ECF osmolarity.
↓
osmoreceptors in supraoptic nuclei of hypothalamus
↓
↓ADH secretion from posterior pituitary.
↓
↓Plasma ADH
↓
↓Permeability of water in DCT, CT & CD in kidney
↓
↓ Water reabsorption
↓
↓ ECF volume.
↓
↑ Na conc. in ECF.
2. Thirst mechanism:
Dehydration
↓
↓Blood volume
↓
↑ Na conc. in ECF
↓
↑ECF osmolarity
↓
thirst center in Hypothalamus
↓
↑ Water drinking
↓
↑ Water in ECF
↓
↓ Nat conc. in ECF.
Again,
Nat conc. in ECF
↓
↓ECF osmolarity
↓
of thirst center
↓
↓Water drinking
↓
↑ Nat conc. in ECF
7. Aldosterone concentration: ↑ aldosterone concentration Nat excretion,
8. Angiotensin II: It Nat excretion by rennin-angiotensin aldosterone mechanism.
9. ANP: It is a natreuretic factor. It Na¹ reabsorption from PCT & CD.
10. Salt-appetite mechanism: Na+ in the body salt appetite salt gain.
11. Tubular load of Na+: >99% of the filtered Na+ is reabsorbed by the PCT, DT & CD which is regulated by tubule-glomerular and glomerulo-tubular balance mechanism.
12. Sympathetic stimulation: It causes Na* & H₂O retention.
(Ref: Lectures of RMC)
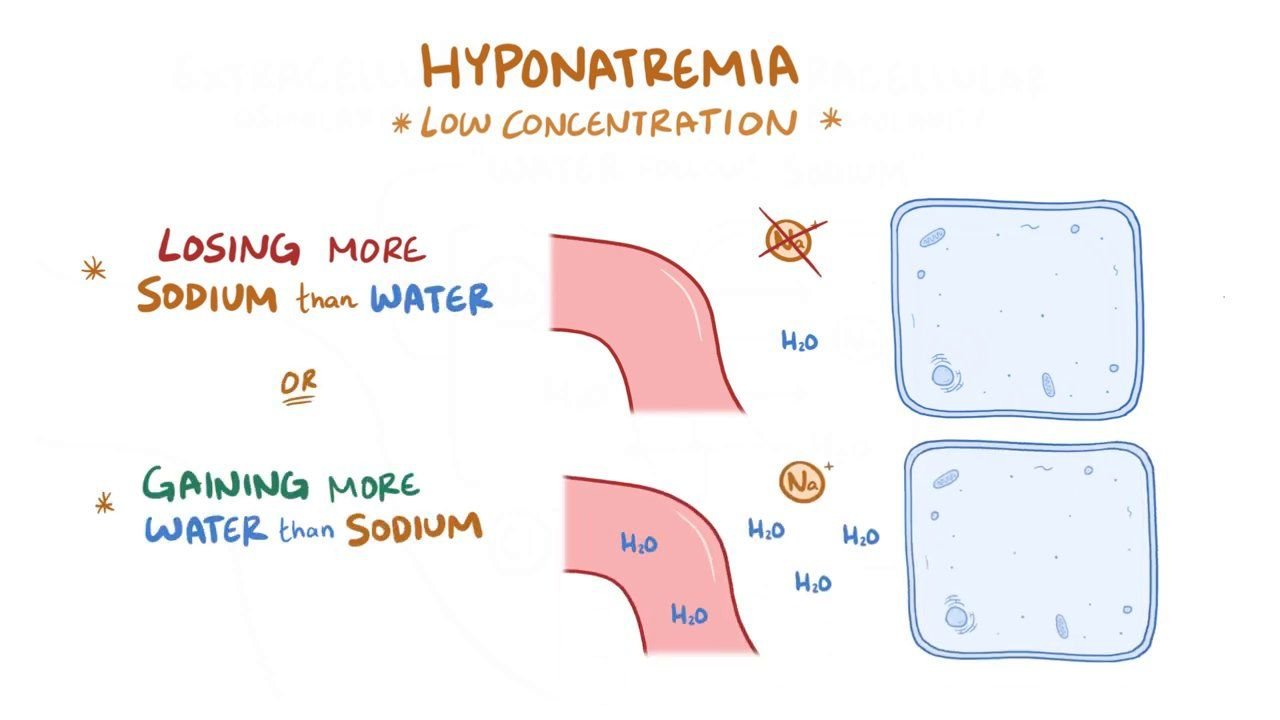
Hyponatremia:
Decreased concentration of Na* in blood or ECF is called Hyponatremia.
Causes of Hyponatremia:
- Renal sodium loss: due to Diuretic therapy, Adenocortical failure (Addison’s disease).
- Gastrointestinal sodium loss: due to Vomiting, Diarrhea.
- Burn.
- Primary polydipsia.
- Syndrome of inappropriate ADH secretion (SIADH).
- Excessive infusion of electrolyte free soln. (e.g 5% glucose soln.).
- Hypothyroidism.
- Nephrotic syndrome.
- Cirrhosis of Liver.
- Chronic renal failure.
In another way
Causes of Hyponatremia:
1. Isotonic hyponatremia:
➤ Hyperproteinemia.
➤ Hyperlipidemia.
2. Hypotonic hyponatremia:
➤ Congestive cardiac failure.
➤ Nephrotic syndrome,
➤ Liver cirrhosis.
➤ Liver failure
3. Hypertonic hyponatremia:
➤ Diabetes mellitus.
➤ Acute renal failure.
4. Iatrogenic cause: If the patient is treated with a lot of hypotonic saline.
5. SIADH (syndrome of inappropriate ADH secretion).
6. Addison’s disease.
7. Diarrhea, vomiting, profuse sweating
8. Excessive use of diuretics.
Hypernatremia:
↑ Na+ concentration in blood above the normal level is called hypernatremia.
Causes of Hypernatremia:
➤ Hyperactivity of the adrenal cortex as in Cushing syndrome.
➤ Prolong treatment with Cortisone, ACTH,,Sex hormone.
➤ Excessive improperly diluted ORS intake.
➤ Diabetes insipidus.
➤ Chronic renal failure.
Hypokalemia
↓K+ concentration in blood below the normal level is called hypokalemia.
Causes of Hypokalemia:
- Prolong diarrhea & vomiting.
- Prolonged use of diuretics.
- Prolong corticosteroid therapy.
- Severe PEM
- Heart failure (treatment with digitalis).
Hyperkalemia:
↑ K+ concentration in blood above the normal level is called hyperkalemia.
Causes of Hyperkalemia:
- Renal failure.
- Severe dehydration.
- Excessive intake of potassium salts.
- Hemolysis.
- Crush injury.
- Insulin deficiency.
- Drugs: e.g. B-blocker, NSAIDs, ACE inhibitors, Spironolactone etc.
Effect of hypernatremia:
- Thirst
- Mild confusion
- Disorientation
- Depress the cardiac function
- Muscular weakness
Effect of hyponatremia:
- Lassitude.
- Sleepiness
- Myoclonic jerks.
- Generalized seizures.
Effect of hyperkalemia:
- Tingling around the lips.
- Arrhythmia
- Slow heart rate
- ECG changes-
✓ ‘QRS’ complex is broad & slurred.
✓ ‘T’ wave is tall & slender
Effect of hypokalemia:
- Tiredness
- Muscular weakness
- Generalized weakness
- Tingling in the fingers
- Apathy
- Paralysis.
- Coma
- ECG changes-
✓ ‘P-R’ interval is lengthened
✓ ‘ST’ segment is depressed.
✓ ‘T’ wave is inverted.
Anion gap:
The difference between the unmeasured cation and the unmeasured anion in the plasma is called
Anion gap.
Shortly, it is the gap between the cation and anion in the plasma.
So, Anion gap Unmeasured cation – Unmeasured anion.
Normally anion gap is about 12 meq/L.
The concentration of anions & cations in plasma must be equal to maintain electrical neutrality.
So total anoin = total cation.
Or, Measured anion (MA) + Unmeasured anion (UA)= Measured cation (MC) + Unmeasured
cation (UC)
Or, MA+UA=MC+UC
Or, UA-UCMC-MA
Or, UA-UC = (Na+ + K’) (HCO3 + Cl) [measured means routinely measured in clinical
laboratory]
Or, Anion gap = (104+4)-(24+108) = 12 meq/L.
Measured anion: HCO3, CI.
Unmeasured anion: Albumin, Phosphate, Sulphate, Lactate etc.
Measured cation: Na+, K+
Unmeasured cation: Ca++, Mg++.
Anion gap is increased in –
➤ Diabetic ketoacidosis.
➤ Lactic acidosis.
➤ Chronic renal failure.
➤ Aspirin poisoning.
➤ Starvation.
Anion gap is decreased in –
– When plasma cations are increased e.g. Hypercalcemia, Hypermagnesaemia.
– When plasma albumin is decreased (hypoalbuminaemia).
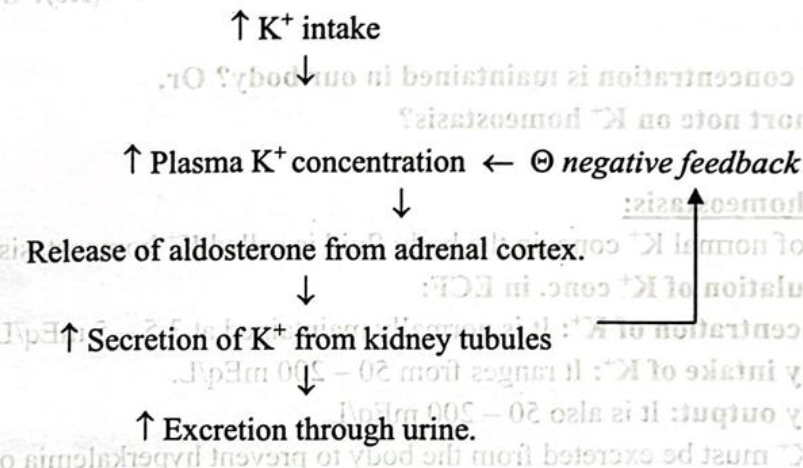
Potassium homeostasis:
Regulation of normal K+ conc. in the body fluid is called K+ homeostasis.
➤ Regulation of K+ conc. in ECF:
➤ Concentration of K+: It is normally maintained at 3.5-5 mEq/L.
➤ Daily intake of K⁺: It ranges from 50-200 mEq/L.
➤ Daily output: It is also 50-200 mEq/L.
So, excess K must be excreted from the body to prevent hyperkalemia or conserve K+ to prevent hypokalemia.
Kidney plays an important role in K+ excretion from the body. Only about 5-10% of the intake K¹ is excreted through faeces
Factors affecting plasma K+ concentration:
1. Intestinal handling of K+:
➤ Entry: Exogenous (foods) & Endogenous (digestive juices) K+.
➤ Absorption: 140-150 mmol/day.
➤ Excretion: 10-20 mmol/day.
2. Renal handling of K+:
➤ Tubular load of K+: 700-800 mmol/day.
➤ Tubular reabsorption: 98%
➤ Tubular secretion: 80-90 mmol/day
Factors that regulate internal K+ distribution:
| Factors that shift K+ into the cells (extracellular K+) | Factors that shift K+ out of the cells (Textracellular K¹) |
| i. Insulin. | i. Insulin deficiency |
| ii. Aldosterone. | ii. Aldosteron deficiency. |
| iii. Epinephrine and Nor epinephrine. | iii. ẞ-adrenergic blocker |
| iv. Alkalosis. | iv. Acidosis. |
| v. Cell lysis. | |
| vi. Strenuous exercise, |
Feedback control of K+ concentration:
NOTE
Sources of K+:
➤ Exogenous: Banana, Green coconut water, Grapes, Apples, Guava etc.d
➤ Endogenous: Digestive juice specially bile & pancreatic juice.
Route of K+ excretion:
➤ Urine.
➤ Stool.
➤ Sweating.
K+ concentration must be maintained within a narrow range 3.5-5 mmol/L, if not, the person will die due to cardiac arrest.

