Formation of Ions – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Formation of Ions
Definition of Ionic Bonds:
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds.
or
Bond in which one or more electrons from one atom are removed and attached to another atom, resulting in positive and negative ions which attract each other is known as ionic bond.
Process of Formation of Ionic Bonds
An ionic bond is formed through the transfer of one or more valence electrons, typically from a metal to a non-metal, which produces a cation and an anion that are bound together by an attractive electrostatic force. On a macroscopic scale, ionic compounds, such as sodium chloride (NaCl), form a crystalline lattice and are solids at normal temperatures and pressures.
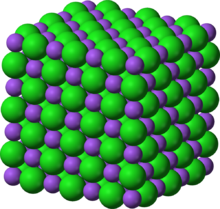
Crystalline Lattice: Sodium chloride crystal lattice
The charge on the cations and anions is determined by the number of electrons required to achieve stable noble gas electronic configurations. The ionic composition is then defined by the requirement that the resulting compound be electrically neutral overall.
For example, to combine magnesium (Mg) and bromine (Br) to get an ionic compound, we first note the electronic configurations of these atoms (valence level in indicated in italics):
- Mg: 1s ^ 2 * 2s ^ 2 * 2p ^ 6 * 3s * 2
- Br: 1s ^ 2 * 2s ^ 2 * 2p ^ 6 * 3s ^ 2 * 3p ^ 6 * 3d ^ 10 * 4s * 24ps
In order to achieve noble gas configurations, the magnesium atom needs to lose its two valence electrons, while the bromine atom, which has 7 valence electrons, requires one additional electron to fill its outer shell. Therefore, for the resulting compound to be neutral, two bromine anions must combine with one magnesium cation to form magnesium bromide (MgBr₂).
In addition, though any ratio of 2 bromine atoms to 1 magnesium atom will satisfy the two requirements above, the formula for ionic compounds is typically presented as the empirical formula, or the simplest whole-number ratio of atoms with positive integers.
Note that the cation always precedes the anion both in written form and in formulas. In the written form, while the cation name is generally the same as the element, the suffix of single- atom anions is changed to ide, as in the case of sodium chloride. If the anion is a polyatomic ion, its suffix can vary, but is typically either -ate or -ite, as in the cases of sodium phosphate and calcium nitrite, depending on the identity of the ion.