Ionic Bonds – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection and disposal. The aim of the course is to acquire knowledge and skills in general biological science, general chemistry and physics.
Ionic Bonds
Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ions. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. Ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal.
Ionic bonding is observed because metals have few electrons in their outer-most orbitals. By losing those electrons, these metals can achieve noble gas configuration and satisfy the octet rule. Similarly, nonmetals that have close to 8 electrons in their valence shells tend to readily accept electrons to achieve noble gas configuration.
In ionic bonding, more than 1 electron can be donated or received to satisfy the octet rule. The charges on the anion and cation correspond to the number of electrons donated or received. In ionic bonds, the net charge of the compound must be zero.
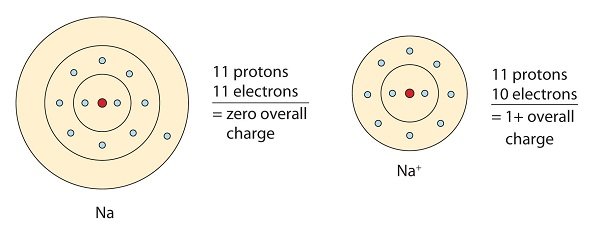
This sodium molecule donates the lone electron in its valence orbital in order to achieve octet configuration. This creates a positively charged cation due to the loss of electron.

This chlorine atom receives one electron to achieve its octet configuration, which creates a negatively charged anion.
The predicted overall energy of the ionic bonding process, which includes the ionization energy of the metal and electron affinity of the nonmetal, is usually positive, indicating that the reaction is endothermic and unfavorable. However, this reaction is highly favorable because of the electrostatic attraction between the particles.
At the ideal interatomic distance, attraction between these particles releases enough energy to facilitate the reaction. Most ionic compounds tend to dissociate in polar solvents because they are often polar. This phenomenon is due to the opposite charges on each ion.
Example: Chloride Salts
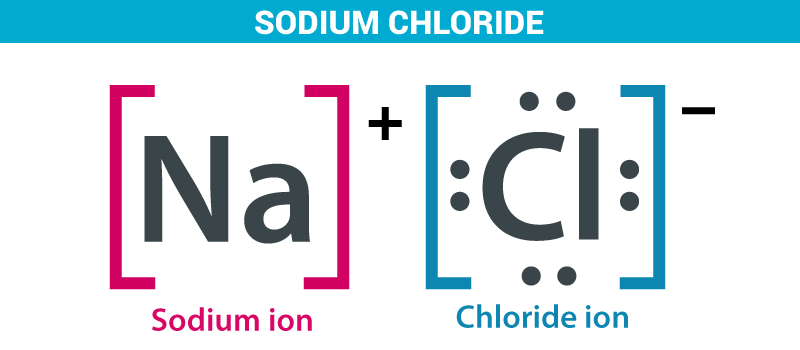
In this example, the sodium atom is donating its 1 valence electron to the chlorine atom. This creates a sodium cation and a chlorine anion. Notice that the net charge of the resulting compound is 0.
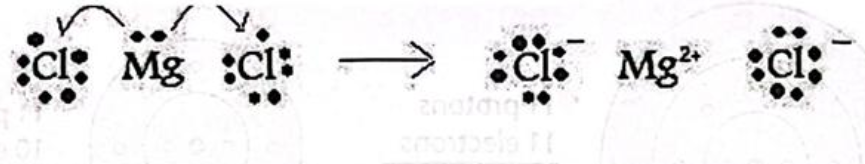
In this example, the magnesium atom is donating both of its valence electrons to chlorine atoms. Each chlorine atom can only accept 1 electron before it can achieve its noble gas configuration; therefore, 2 atoms of chlorine are required to accept the 2 electrons donated by the magnesium. Notice that the net charge of the compound is 0.