State of Matter – Introduction to fundamental concepts of Biological Science including the organization and common characteristics of living matters, cell structures and functions, food production by photosynthesis, harvesting energy, mechanism of cells reproduction, genetics, evolutions, and Human Biology. Introduction to general chemistry including basic concepts about matter, atomic structure, chemical bonds, gases, liquid, and solids, solutions, chemical reactions, acid, bases, and salt;
organic and biochemistry including hydrocarbons and their derivatives, carbohydrates, lipids, proteins, enzymes, vitamins, and minerals, nucleic acids; principles of physics and applications to nursing including gravity and mechanics, pressure, heat and electricity; nuclear chemistry and nuclear physics, effects of radiation on human beings, and protection a
State of Matter
A state of matter is defined as one of the ways in which matter can interact with itself to form a homogeneous phase.
Examples: Solid, Liquids, Gases, Plasma
The three states of matter are the three distinct physical forms that matter can take in most environments: solid, liquid, and gas. In extreme environments, other states may be present, such as plasma, Bose-Einstein condensates, and neutron stars. Further states, such as quark-gluon plasmas, are also believed to be possible. Much of the atomic matter of the universe is hot plasma in the form of rarefied interstellar medium and dense stars.
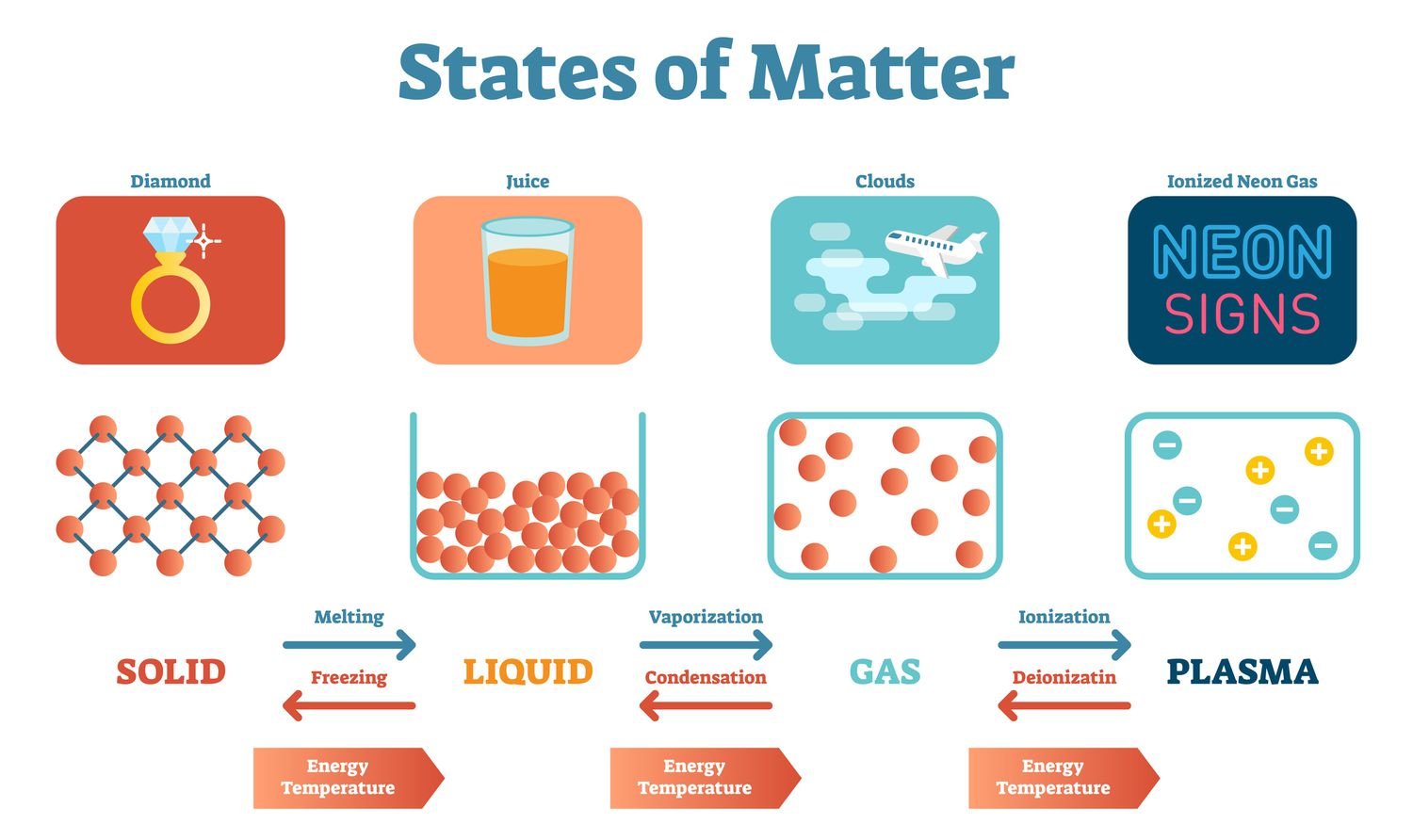
he states of matter: This diagram shows the nomenclature for the different phase transitions.
Solids
- A solid has a definite shape and volume because the molecules that make up the solid are packed closely together and move slowly. Solids are often crystalline; examples of crystalline solids include table salt, sugar, diamonds, and many other minerals. Solids are sometimes formed when anA liquids or gasses are cooled; ice is an example of a cooled liquid which has become solid. Other examples of solids include wood, metal, and rock at room temperature
Liquids
- A liquid has a definite volume but takes the shape of its container. Examples of liquids include water and oil. Gasses may liquefy when they cool, as is the case with water vapor. This occurs as the molecules in the gas slow down and lose energy. Solids may liquefy when they heat up molten lava is an example of solid rock which has liquefied as a result of intense heat
Gases
- A gas has neither a definite volume nor a definite shape. Some gasses can be seen and felt, while others are intangible for human beings. Examples of gases are air, oxygen, and helium. Earth’s atmosphere is made up of gases including nitrogen, oxygen, and carbon dioxide
Plasma
- Plasma has neither a definite volume nor a definite shape. Plasma often is seen in ionized gases, but it is distinct from a gas because it possesses unique properties. Free electrical charges (not bound to atoms or ions) cause the plasma to be electrically conductive. The plasma may be formed by heating and ionizing a gas. Examples of plasma include stars, lightning, fluorescent lights and neon signs
Nice To Know
Chemistry Term:
- Fusion/Melting
- Freezing
- Vaporization/Boiling
- Condensation
- Sublimation
- Deposition
Phase Change:
- Solid to a liquid
- Liquid to a solid
- Liquid I to a gas
- Gas to a liquid
- Solid to a gas
- Gas to a solid