Today our topic of discussion is ” Water Balance “. Water is the most abundant and important medium in the human body, vital for maintaining the structure and function of biological systems. It plays a crucial role in the body’s fluid, electrolyte, and acid-base balance, influencing cellular function, blood volume, and the overall homeostasis of the organism. This article explores the intricate system of water balance and its interrelation with fluid, electrolyte, and acid-base balance.
Water Balance : Fluid, Electrolyte, and Acid-Base Balance
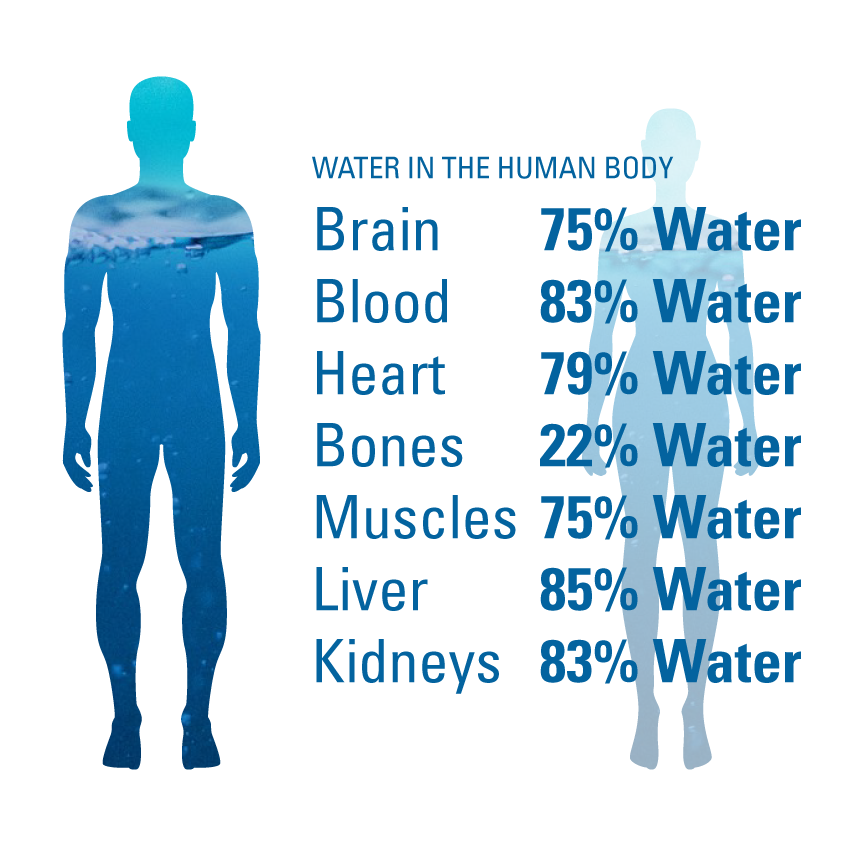
Understanding Water in the Human Body
The human body is composed of approximately 60% water, which varies according to body composition and age. Water is distributed in two main compartments within the body: intracellular fluid (ICF), which is the fluid within cells, and extracellular fluid (ECF), which includes interstitial fluid, blood plasma, and transcellular fluid.
Functions of Water in the Body
- Solvent: Water acts as a solvent, dissolving electrolytes, nutrients, and waste products.
- Transport Medium: It transports substances to and from cells and within the bloodstream.
- Thermal Regulation: Water has a high heat capacity, helping to regulate body temperature.
- Lubrication: It lubricates joints and acts as a shock absorber within the eyes, spinal cord, and amniotic sac in pregnancy.
- Chemical Reactions: Water participates in hydrolytic reactions and helps in maintaining pH balance.
Water Balance Mechanisms
Water balance is tightly regulated to ensure that the amount of water consumed and metabolized is equal to the amount excreted. The following are the primary mechanisms by which the body maintains water balance:
Intake
Average water intake occurs through drinking fluids and eating foods, as well as from metabolic processes. The hypothalamus in the brain detects changes in plasma osmolality (concentration of solutes in the blood) and stimulates thirst when water intake needs to be increased.
Output
Water is lost from the body through urine, feces, sweat, and exhalation. The kidneys play a crucial role in water balance by adjusting urine output. The loss of water is regulated by antidiuretic hormone (ADH), which acts on the kidneys to conserve water when necessary.
Fluid Balance
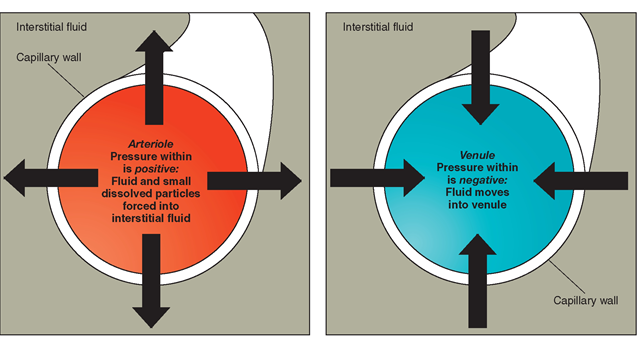
Fluid balance refers to maintaining the volume and composition of the body’s fluid compartments. The movement of water and solutes between these compartments is governed by osmosis, diffusion, and active transport.
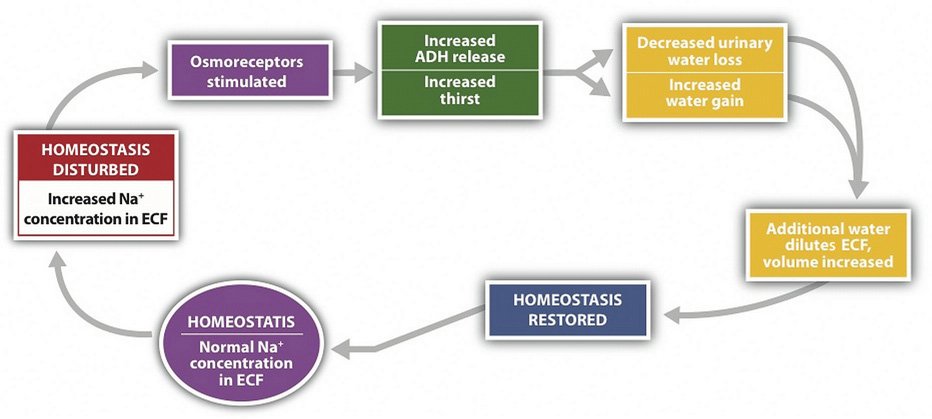
Osmoregulation
Osmoregulation is the process by which the body maintains the osmotic pressure of body fluids within a narrow range. Osmotic pressure is determined by solutes such as sodium, potassium, and chloride. When osmotic pressure is high, it prompts the movement of water into the blood, increasing blood volume and blood pressure. Conversely, low osmotic pressure leads to water moving out of the bloodstream, potentially resulting in dehydration.
Electrolyte Balance
Electrolytes like sodium (Na+), potassium (K+), calcium (Ca2+), and magnesium (Mg2+) are vital for various body functions. They are involved in nerve impulse transmission, muscle contraction, and maintaining the electric potential across cellular membranes. The balance of electrolytes is closely tied to water balance since they are dissolved in body fluids and influence osmotic pressure.
Acid-Base Balance
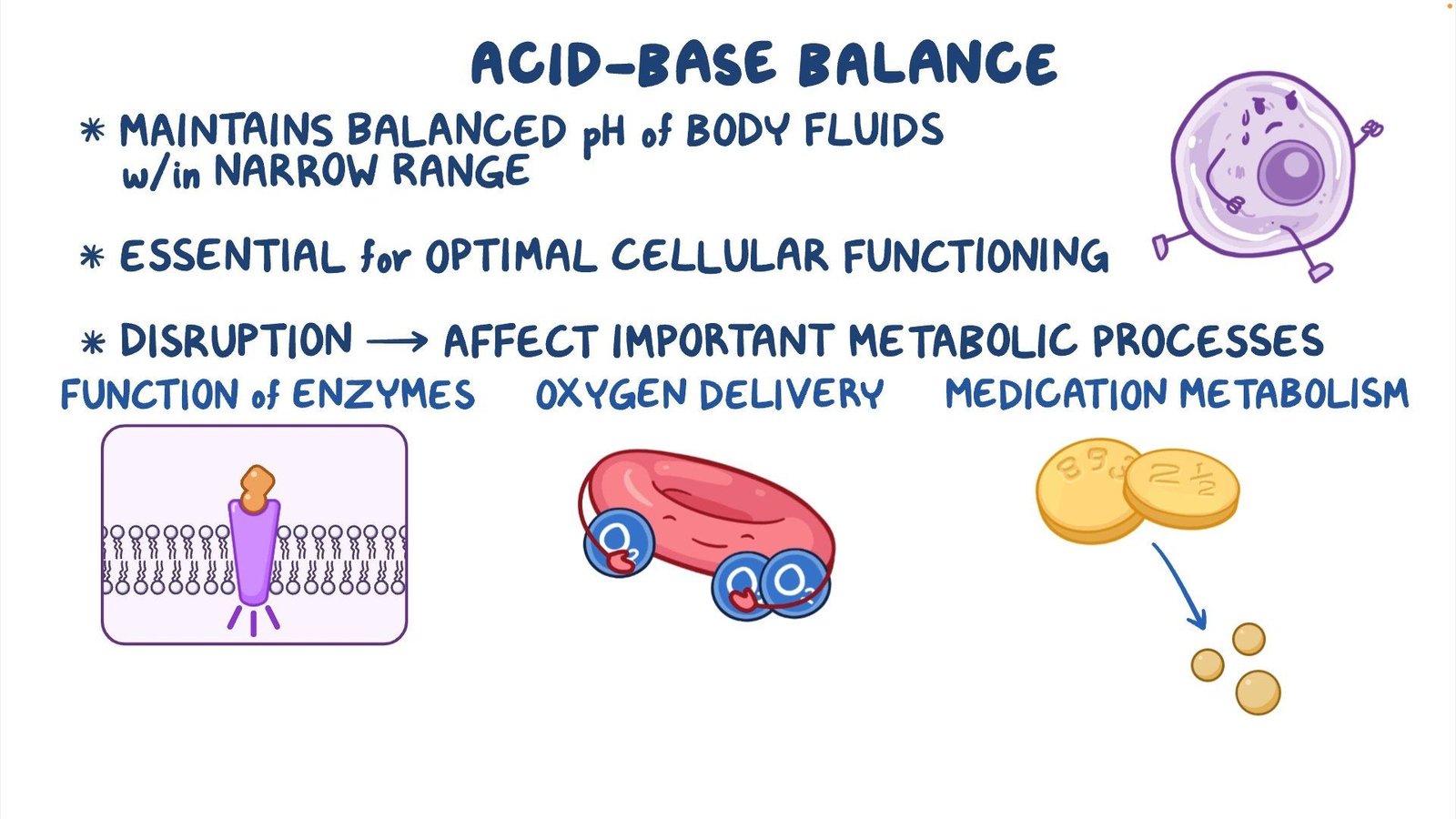
Acid-base balance is the homeostatic regulation of the body’s pH levels. The body’s fluids need to maintain a slightly alkaline pH of 7.35-7.45. The three major mechanisms that regulate acid-base balance are:
- Buffer Systems: These are the first line of defense against pH changes and include bicarbonate, phosphate, and protein buffer systems.
- Respiratory Compensation: CO2 is a byproduct of metabolism and can combine with water to form carbonic acid (H2CO3), which dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-). The respiratory system regulates CO2 levels, thus influencing pH balance.
- Renal Compensation: The kidneys excrete or reabsorb H+ and HCO3- to maintain the body’s pH balance. They can also produce ammonia (NH3) to buffer H+ ions in the urine.
Disorders of Water Balance
Disorders of water balance can significantly impact fluid, electrolyte, and acid-base balance. These include:
Dehydration
Dehydration occurs when water output exceeds water input. Causes include vomiting, diarrhea, excessive sweating, or inadequate water intake. Symptoms include thirst, dry skin, fatigue, and in severe cases, hypotension and tachycardia.
Overhydration
Overhydration, or water intoxication, occurs when there is an excess of water in relation to electrolytes, leading to hyponatremia (low sodium levels). It can cause cells to swell, resulting in neurological symptoms such as headache, confusion, and in severe cases, seizures and coma.
Electrolyte Imbalance
Electrolyte imbalances, such as hyponatremia, hypernatremia (high sodium levels), hypokalemia (low potassium levels), or hyperkalemia (high potassium levels), can have serious consequences. Symptoms range from muscle weakness and cramps to cardiac arrhythmias and neurological disturbances.
Acidosis and Alkalosis
Acidosis is an excess of H+ ions, leading to a decrease in blood pH. Alkalosis is the opposite, with a deficiency of H+ ions increasing blood pH. Both conditions can be respiratory or metabolic in origin and can affect cellular function and metabolism.

Maintaining Water Balance
Maintaining water balance requires adequate intake and proper functioning of regulatory mechanisms. Guidelines suggest that adult men need about 3.7 liters and adult women about 2.7 liters of water from all beverages and foods each day, but individual needs can vary greatly based on factors like climate, physical activity, and health status.
In addition to consumption, factors such as medications, diet (particularly salt intake), and lifestyle choices (such as alcohol consumption) can influence water balance.
Conclusion
Water balance is a complex interplay of various systems and processes in the body. It is a fundamental aspect of maintaining life, intricately connected with fluid, electrolyte, and acid-base balance. A delicate equilibrium must be maintained to ensure physiological functions are not compromised. Understanding the importance of water balance and the mechanisms involved is crucial for healthcare professionals and individuals alike to promote optimal health and prevent related disorders.
Read more:
